Owing to the high diffusion rates of base (solid phase) metal atoms in a liquid, alloying additions that form a liquid phase during the process often result in high densification rates, lower sintering temperatures, and greater cost effectiveness. Liquid-phase sintering (LPS) of refractory metals usually involves the addition of transition metals such as Ni (as elemental powders) that have high solubility for the refractory metal and form a thermodynamically stable second phase with a liquidus temperature below 1500℃.
Liquid-phase sintering has traditionally been broken down into initial, intermediate, and final stages. The initial stage, rearrangement, begins with the formation of the liquid phase. As the liquid forms an increase of the refractory metal, solubility in the liquid phase enables dissolution of the solid-solid contacts that form during heating. Capillary forces due to the wetting liquid act on the solid particles and pull them together, resulting in rapid shrinkage. In the intermediate stage, solution-reprecipitation, atoms at particle contacts and other convex points dissolve in the liquid phase and diffuse to neighboring concave surfaces where they reprecipitate. As the grains change shape, they pack better and release liquid to fill any remaining pores. In addition to densification, the solution-reprecipitation process also causes grain growth via Ostwald ripening. The final stage in LPS involves continued microstructural coarsening with slower densification because of the rigidity of the solid skeleton. This stage of LPS is generally avoided in practice, particularly for the MIM process where prolonged heating can result in distortion and loss of shape retention. By this stage, densification is practically complete and further increases in grain size can degrade properties.
Most densification of heavy alloys occurs prior to liquid phase formation. Nickel and Fe additions, in particular, enhance the solid-state sintering of W, as discussed in Section 23.5.2. A plot showing densification as a function of temperature for W-8.4Ni-3.6Fe and W-8.4Ni-3.6Cu is given in Fig. 23.4. For W-Ni-Fe, the liquid phase begins forming at 1400℃, and full densification is mostly achieved before the matrix fully melts at 1455℃. W-Ni-Cu is less densified when the first liquid phase forms at 1285℃, but then densifies rapidly with a second increase in densification rate occurring when the matrix fully melts at 1370℃.
Fig. 23.4 Densification of W-Ni-Fe and W-Ni-Cu heavy alloys during heating at a rate of 10℃/min
Once the liquid forms, the rate of grain growth increases significantly as shown in Fig. 23.5. Because densification and grain growth of heavy alloys occur so rapidly when the liquid phase forms, they are less sensitive to the initial particle size than solid-state sintered materials. During isothermal sintering, the grain size increases in proportion to the cube root of time. The proportionality factor is called the grain-growth-rate constant and depends on the diffusivity and solubility of the W in the matrix and the volume fraction of the matrix. Higher W volume fractions result in higher grain-growth-rate constants, as shown in Fig. 23.6. The matrix volume fraction has only a minor effect on densification up to about 97 wt% W but can significantly affect distortion, as discussed later.

Fig. 23.5 Increase in grain size for 88W-8.4Ni-3.6Fe during heating up to 1500℃ and during isothermal sintering at 1500℃. The hold times are noted. The liquid forms between 1400 and 1500℃.
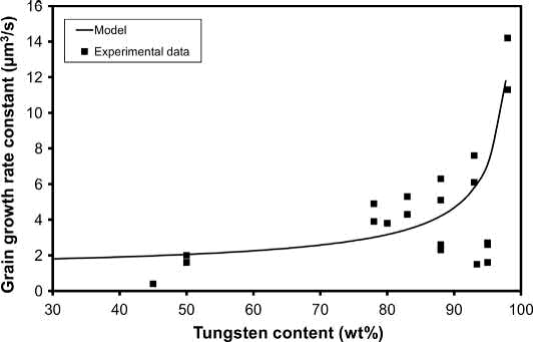
Fig. 23.6 Effect of tungsten content on the grain-growth-rate constant for LPS W-Ni-Fe heavy alloys
Typical process cycles
Heavy alloys are typically sintered in furnaces that are capable of 1500℃ in pure hydrogen or hydrogen/nitrogen mixtures such as dissociated ammonia. Typical configurations are continuous pusher-type furnaces; however, vacuum furnaces can also be used. Dry hydrogen can be used; however, wet hydrogen has become the industry standard to suppress both blister defect formation and hydrogen embrittlement. The entire process can be performed under wet hydrogen or a process that initially involves dry hydrogen prior to pore closure to obtain the maximum oxide reduction and subsequent wet hydrogen prior to pore closure and liquid formation to prevent the previously mentioned defects. Using wet hydrogen later in the sintering cycle can minimize the amount of trapped water vapor.
The process cycle for these alloys consists of a low-temperature hold in the 900-1100℃ range to obtain maximum oxide reduction and outgassing of volatiles from the powder surface prior to pore closure. Reduction of the metal oxides before pore closure is important; otherwise, water vapor can become trapped, causing residual porosity, blistering, and hydrogen embrittlement. A subsequent increase to a sintering temperature in the 1325-1500℃ range with a hold of 20min to 2 h is recommended. The time/temperature profile is a function of the alloy, desired microstructure, and size of the component. Higher sintering temperatures and longer sintering holds are recommended for higher W content alloys, the nickel-iron alloys, and property sensitive components. Higher sintering temperatures, longer sintering times, and lower W contents lead to distortion as the part slumps under its own weight. Lower temperatures and shorter holds are recommended for lower W contents, nickel-copper alloys, and dimensionally sensitive components.
Micrographs of MIM microstructures are shown in Figs. 23.7 and 23.8. The grains are fully rounded in Fig. 23.7 and are not fully rounded in Fig. 23.8. The slightly undeveloped microstructure of Fig. 23.8 is characteristic of a MIM component that requires superior feature definition. If a fully developed microstructure, as shown in Fig. 23.7, is required for particular properties, a lack of feature definition should be expected. Shape retention can also be obtained by utilization of a greater tungsten content alloy, i.e. the use of 95 wt% W as opposed to 90 wt% W.
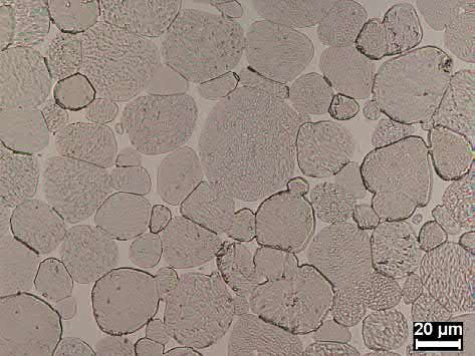
Fig. 23.7 95W-4Ni-1Fe MIM heavy alloy sintered at 1490℃ for 1 h in hydrogen gas, 35μm grain size
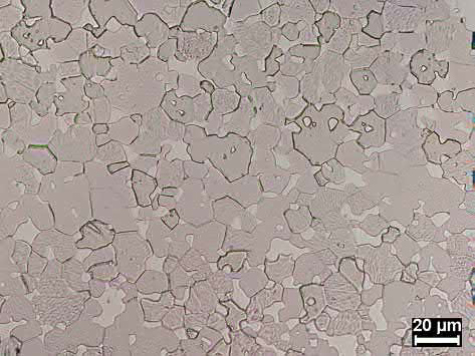
Fig. 23.8 95W-4Ni-1Fe MIM heavy alloy sintered at 1480℃ for 1 h in hydrogen gas, 10μm grain size
The cooling rate also influences the final properties. A slow cooling rate reduces the amount of solid solution W in the matrix but can also result in intermetallic formation depending on the alloy composition. W-Ni-Fe alloys consisting of 90-97 wt% W are usually sintered to near full density in hydrogen at 1470-1580℃. Substituting Cu for Fe lowers the sintering temperature of the tungsten-based alloys to a range similar to the sintering temperatures of 316L or 17-4PH stainless steels. These W-Ni-Cu alloys are sintered to near full density in dry hydrogen at temperatures between 1325℃ and 1380℃. W-Ni-Cu alloys with lower Ni:Cu ratios form a liquid phase at lower temperatures but have poorer densification behavior due to the lower solubility of W in the matrix.
Distortion effects
The high density and the liquid phase that assists densification of heavy alloys can also lead to poor dimensional stability unless sufficient contacts form between the solid grains to resist gravitational, surface tension, and frictional forces. The resistance of a LPS MIM component to distortion by these forces is determined by its viscosity during sintering. Higher amounts of liquid and larger grain sizes result in lower bulk and shear viscosities and less dimensional stability (Park, Chung, et al., 2006). To avoid slumping, LPS is primarily limited to compositions with high solid volume fractions. W-Ni-Fe heavy alloys generally display distortion for W contents <90wt%, corresponding to a solid volume fraction of about 0.76. Heavy alloys with W contents of 90wt% or above can also slump with high sintering temperatures or long sintering times, which also results in microstructural coarsening, and property and feature fidelity degradation.
Finite element modeling can accurately predict the effects of gravity, surface tension, substrate friction, solid content, and sintering time on the shapes of sintered components. Features to avoid with heavy alloys are cantilevers or large poorly supported sections. Larger MIM components are more susceptible to slumping due to gravity and to friction-related distortion due to sticking of the part to the substrate, while surfacetensioncan cause rounding of features onmicro-MIM parts.Fig. 23.9 shows microstructures of (A) properly sintered and (B) an oversintered heavy alloy. In the case of oversintering, the liquid phase begins to remove features owing to wetting effects at fine feature detail.
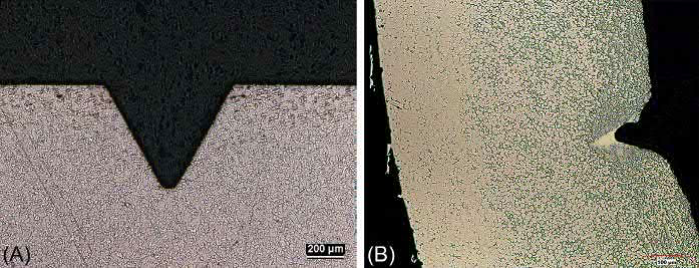
Fig. 23.9 (A) Properly sintered. (B) Oversintered 90W-8Ni-2Fe MIM features. The oversintered feature shows the liquid filling the feature and rounding of the outside corners.
Practical process concerns
The primary concerns when processing heavy alloys by MIM are microstructural uniformity/full development, distortion due to slumping, shape retention, and blistering due to an inappropriate sintering atmosphere and/or profile.
Microstructure can be affected by each process step. If the powders are not fully blended or milled, the microstructure could have regions of liquid-phase-forming elements which reduce the local properties. This microstructural behavior can also be caused by poorly deagglomerated refractory metal powder or by green state cracks that fill with liquid during sintering, giving enriched regions of refractory metal and liquid-phase-forming metal. Typically, poor milling or poor blending cause pools of liquid that are globular, whereas green state cracks produce pools of liquid that are linear in nature. Fig. 23.10 shows a micrograph of this effect for a green state crack.
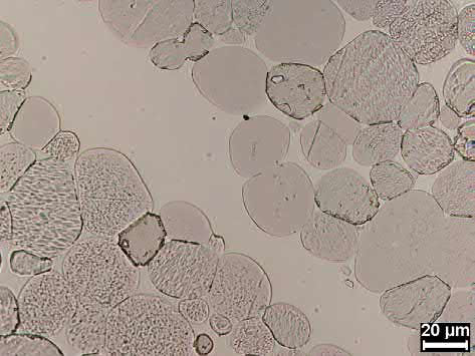
Fig. 23.10 Liquid-filled region of a tungsten heavy alloy, which had a green state crack tha filled with liquid during sintering
Distortion, which is characterized by slumping and loss of feature shape retention, occurs for all MIM systems and is primarily a function of geometry, material viscosity at sintering temperature, and density. Since heavy alloys have the greatest density of any MIM material and are liquid-phase sintered, they have the greatest propensity to distort. This distortion can be mitigated by using several techniques. The microstructure can be undersintered which minimizes the amount of liquid during sintering. This, however, results in an undeveloped microstructure which may have inferior mechanical properties, but nominal density properties and excellent dimensional consistency. Another technique is to change the alloy to a higher quantity of solid phase, which will shift the properties, but allow for a fully developed microstructure and acceptable dimensional consistency. The final technique to reduce distortion is by the use of setters to properly support the components. Setters for heavy alloys are typically fabricated from a fiber or high alumina. This setter would be designed to support the component and minimize gravity-induced distortion or slumping. Selection of components that have minimal unsupported sections can also prevent distortion in this class of materials.
Blistering of MIM heavy alloys can be a significant issue in dry hydrogen. The primary method to eliminate these defects is to sinter in wet hydrogen to suppress the formation of water vapor voids in the microstructure, which coalesce at elevated temperatures and erupt at the surface of the component.
Tungsten heavy alloys are of engineering interest due to their uniquely high density and mechanical properties. These alloys are capable of 19 g/cm3 density, 35% elongation, 43 HRC hardness, and 1380MPa (200 ksi) ultimate tensile strength. Table 23.4 provides an overview of some typical properties for different classes of heavy alloys, which are based on W weight percentage.
These alloys are susceptible to hydrogen embrittlement and are exposed to hydrogen during the sintering operation, thus hydrogen outgassing can be required to enhance the ductility of the alloy. Typically, solution annealing at 900-1300℃ and subsequent quenching can avoid impurity segregation and formation of intermetallic phases. Table 23.5 shows a study performed on a 95 wt% W alloy that received vacuum treatment. The material was sintered at two temperatures in pure hydrogen-1480℃ and 1490℃. After sintering, half of the samples were vacuum annealed at 1200℃ for 2 h in a 10-6 Torr vacuum.
Table 23.4 Typical heavy alloy properties
90W6Ni | 90W7Ni | 95W3.5Ni | 95W3.5Ni | 97W2.1Ni | |
Alloy | -4Cu | -3Fe | -1.5Cu | -1.5Fe | -0.9Fe |
ASTM-B-777-15 Typical density (g/cm3) Hardness (RC) UTS (MPa) 0.2% YS (MPa) Elongation (%) Elastic modulus (GPa) Thermal expansion (20–400°C, ppm/K) Thermal conductivity [W/(mK)] Electrical conductivity (% IACS) Relative magnetic permeability | Class 1 16.96 24 770 517 6 280 5.4 96 14 <1.01 | Class 1 17.00 25 860 610 15 270 4.8 75 10 5.0-5.5 | Class 3 18.0 27 760 590 5 310 4.4 140 16 <1.01 | Class 3 18.12 29 862 620 3 340 4.6 110 13 4.0-4.5 | Class 4 18.56 30 883 586 2 360 4.5 125 17 1.6-2.0 |
Table 23.5 Effect of vacuum anneal on the mechanical properties of a 95wt.% W heavy alloy
Sintering temperature(°C) | Vacuum anneal | Density (g/cm3) | YS (MPa) | UTS (MPa) | Elongation (%) |
1480 1480 1480 1480 | No Yes No Yes | 18.0 18.1 18.0 18.0 | 598 717 596 641 | 814 968 658 957 | 6.1 17.3 3.7 18.7 |
Refractory metals and refractory metal alloys that are solution or dispersion strengthened are usually solid-state sintered at high temperatures due to their high melting temperatures. They have been traditionally sintered by passing current through the consolidated powder to heat it directly. More current flows through the part as its density increases and its resistivity decreases. Direct sintering can achieve temperatures up to 3000℃ with a few thousand amperes of current. Although this method is suitable for producing bars that will undergo thermomechanical processing for rods, wires, or plates, it is not practical for sintering netshape MIM parts. Thus, MIM refractory metals are typically solid-state sintered using radiant resistance heating, but specific conditions depend on the particular alloy, particle size, and impurities.
For alloys with mutual solubility of the components, e.g., W-Re alloys, densification and homogenization take place simultaneously during sintering. Diffusion may be enhanced if the alloying additions have a lower melting temperature than the base metal, but Kirkendall porosity may result from the differing diffusion rates. For example, Re additions to W can slow densification if its particle size is larger than that of the W, but otherwise it generally promotes densification due to its slightly higher diffusivity.
Additions of insoluble oxides, such as La2O3, Y2O3, CeO2, and HfO2, can have either an enhancing or a retarding effect on the densification behavior, depending on the quantity of particles added, their size, their distribution, their content, and their type. Lanthanum oxide additions to W enhance densification by pinning grain boundaries via the Zener effect to maintain a small grain size.
Densification of refractory metals occurs through solid-state sintering as particles in contact with each other undergo neck growth and their centers approach each other. Several sintering mechanisms can be concurrently active, but at low temperatures, grain boundary diffusion is the dominant densification mechanism of W and other refractory metals. Once the porosity decreases to about 8% and the pores close, lattice diffusion begins to dominate; however, concurrent grain growth slows densification. In practice, densities above 96% oftheoretical are difficultto achieve for solid-state-sintered refractory metals. The sintering shrinkage of all systems displays an asymptotic characteristic as the compact nears full density and can be predicted from models based on master sintering curve (MSC) concepts. These predictions can be used to construct process maps for the effects of particle size, initial density, sintering temperature, and sintering time.
Based on an MSC model, process maps for the effect of W particle size and sintering temperature on density and grain size for an initial density (solids loading) of 55% of theoretical and a sintering time of 600min are given in Fig. 23.11. Even at 2500℃, W particle sizes above 10μm show little densification or grain growth. Maximum densification can be achieved at 2000℃ with particle sizes <2 μm with about a tenfold increase in grain size. Although the plots show that W particles sizes of 0.5μm or smaller can achieve near full density with little grain growth at temperatures of 1500℃ or lower, the poor packing characteristics of such small particles makes achieving a solids loading of 55% very difficult.
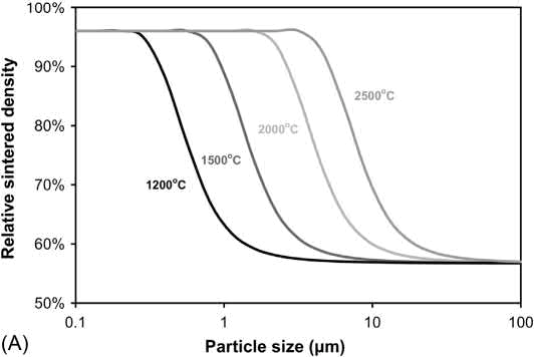
Fig. 23.11 Process maps for the effect of W particle size and sintering temperature on (A) Density
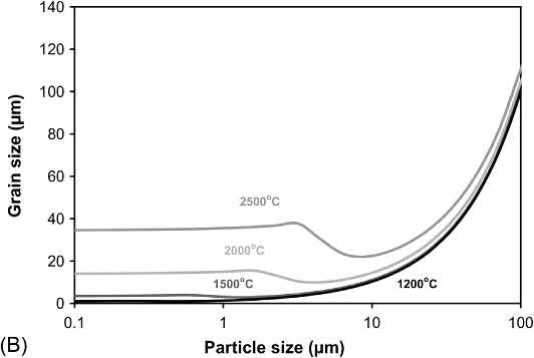
Fig. 23.11-Cont'd (B) Grain size for W powders molded at a solids loading of 55% and sintered for 10 h at the sintering temperature
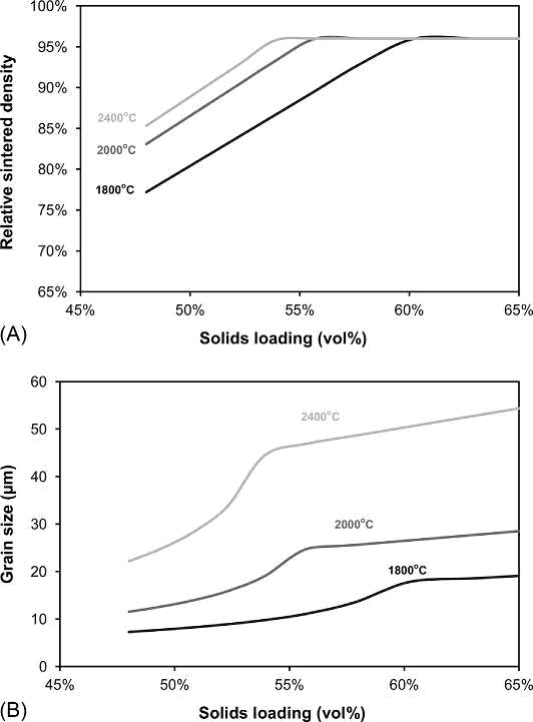
Fig. 23.12 Process maps for the effect of solids loading and sintering temperature on (A) Density. (B) Grain size for a 2.0μm W powder sintered for 10 h at the sintering temperature
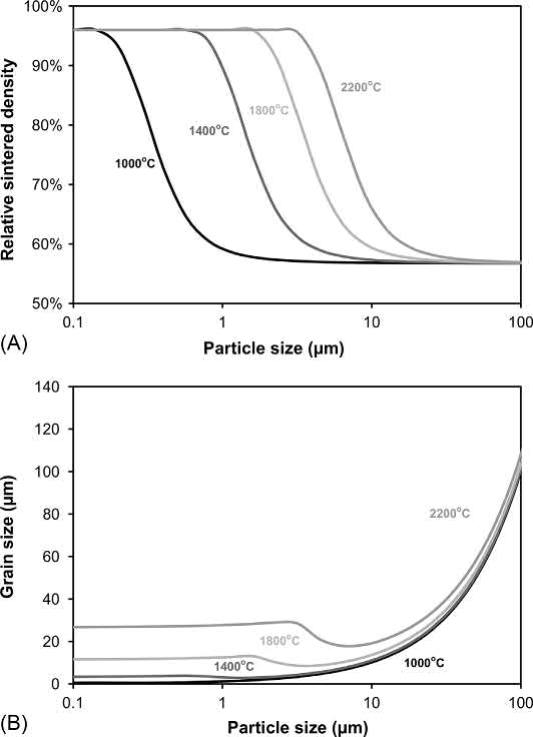
Fig. 23.13 Process maps for the effect of Mo particle size and sintering temperature on (A) Density. (B) Grain size for Mo powders molded at a solids loading of 55% and sintered for 10 h at the sintering temperature
Fig. 23.12 shows the effect of increasing the solids loading on the density and grain size of a 2μm W powder sintered at 1800-2400℃ for 600min. A higher sintering temperature can partially compensate for a low solids loading, but a solids loading of at least 54 vol% is needed to achieve maximum density even at 2400℃. Grain growth becomes significant for solids loadings that enable near full density.
Using the same MSC model, process maps for the effect of Mo particle size and sintering temperature on density and grain size for a solids loading of 55 vol% and a sintering time of 600min are given in Fig. 23.13. The overall behavior is very similar to that of W, although the sintering temperatures are slightly lower due to the lower melting temperature of Mo and its higher diffusivity.
Solid-state sintering can be enhanced or "activated" by small amounts of additives that segregate to grain boundaries and form a high-diffusivity second phase. The most effective sintering activators for W and other refractory metals are transition metals such as Co, Fe, Ni, Pd, and Pt, which have a high solubility for W and relatively low liquidus temperatures but have limited solubility in W. Additions of <1 wt% of these elements greatly reduce the sintering temperature of W and Mo. The sintering of Re can be activated by Pt and Pd additions. Cobalt, Fe, and Ni additions promote the densification of Nb, although niobium oxides can act as a barrier to activated sintering up to 1600℃. Unfortunately, all of the sintering activators are detrimental to properties.
The strong effect of transition metals on the densification and properties of refractory metals necessitates special procedures to ensure that they are not inadvertently picked up from compounding mixers and from the screws and barrels of injection molding machines. These wear surfaces must be properly cleaned if an iron-based material has previously been compounded and molded. Also, the carrier polymers must be properly melted to prevent impurity pick-up from wear of these surfaces. Another concern with the use of these activators, particularly Co, Pt, and Pd, is the degradation of the carrier polymer. These elements act catalytically to break down the polymers even at room temperature. The use of antioxidants as part of the binder system is required to prevent this from occurring.
MIM refractory metal components are typically sintered at temperatures ranging from 1900to 3050℃. The hightemperatures required for sintering require the use of refractory metal setter material. For example, pureW components may be sintered onW sheets. The tendency of refractory metals to oxidize requires reducing atmospheres or vacuum for sintering. In additionto promoting densification,the high sinteringtemperatures help volatilize impurities, such as alkali, earth-alkali, or transition metals, that can significantly reduce the ductility of refractory metals. Heating rates must be slow enough to prevent rapid densification and pore closure before the impurities have been evaporated. For MIM-sized parts, heating rates are generally on the order of 1-4℃/min.
The process cycle for refractory metals includes removal of MIM polymers at temperatures below 600℃ and a subsequent hold in the temperature range of 900-1100℃ to remove oxygen and other residual impurities before heating to the final sintering temperature. One method to obtain sufficient density in a practical fashion is to debind and presinter to a near-closed pore condition in hydrogen to reduce the oxide layer from the particles and to remove other impurities. This is followed by hightemperature sintering in a vacuum. In this way, a higher temperature can be obtained in the vacuum after the hydrogen has reduced the material's particle surfaces.
Tungsten and Mo are usually sintered in 100% hydrogen with a dew point below -20℃. Tungsten components are usually sintered at temperatures of 2000-3050℃ in a flowing dry hydrogen atmosphere to densities of 92%-98% of theoretical with typical grain sizes of 10-30μm. Molybdenum components are usually sintered in flowing dry hydrogen at temperatures ranging from 1700 to 2200℃ to densities of 90%-95% oftheoretical.Reduction ofmolybdenum oxides is important since oxygen contents above 50-200 ppm can cause brittle intergranular failure. Although hydrogen is normally used, vacuum sintering at 1750℃ for 10 h can result in oxygen levels as low as 170 ppm with densities of 97%-98.5% of theoretical and a grain size of about 56μm. An oxygen partial pressure of 75MPa or less is required for the reduction of molybdenum oxides. A typical microstructure of MIM Mo is shown in Fig. 23.14 and typical properties are given in Table 23.6.
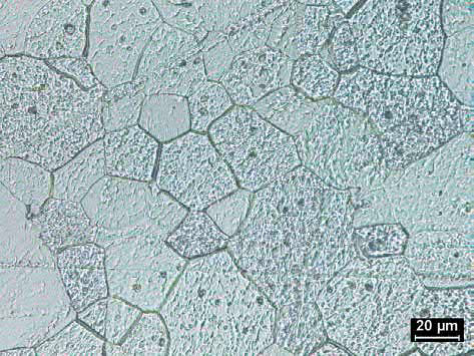
Fig. 23.14 Microstructure of pure molybdenum that was formed by MIM
Table 23.6 Mechanical properties of MIM Mo sintered using a hydrogen reduction up to 1400℃ and vacuum sintering at 2000℃ for 3 h
Material | Density (g/cm3) | Grain size (μm) | UTS (MPa) | Elongation (%) | C (wt.%) | O (wt.%) |
1 2 | 92.8 92.5 | 13 31 | 480 580 | 13 35 | 0.01 0.01 | 0.05 0.19 |
Sintering temperatures for dispersion strengthened W usually range from 2600 to 2800℃, but MIM W with additions of Y2O3, La2O3, TiC, and TaC can be sintered to densities above 98% of theoretical at 2400℃. Mo-CeO2 can be sintered at 1850℃ for 4 h in flowing dry hydrogen but requires hot working for full density. Additions of 3.5 wt% CeO2 decreased the grain size to 1μm from 17μm for unalloyed Mo and increased the yield strength, elongation, and fracture toughness.
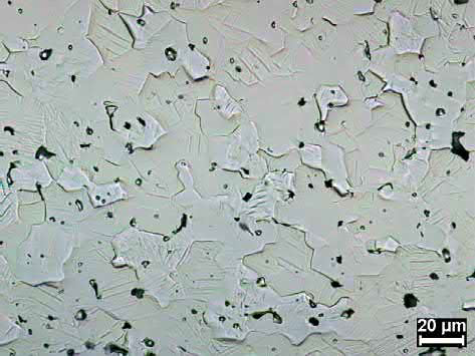
Fig. 23.15 MIM Re sintered at 2400℃, 4 h, in hydrogen and HIPed at 1660℃ and 140MPa
Net-shaped Re components can be presintered at 1200-1400℃ for 30min in hydrogen to increase their handling strength followed by sintering at 2300-2700℃ in hydrogen or high vacuum to densities of 97% of theoretical or higher. Sintered components can also be hot isostatically pressed to help achieve these densities. Grain sizes generally range from 25 to 50μm. An example microstructure is given in Fig. 23.15.
Niobium and Ta form hydrides so sintering is usually performed in a vacuum of 104Torr or better, but can be also done in an inert atmosphere, such as Ar. They are much less tolerant of impurity pick-up than other refractory metals. Tantalum components are usually sintered in a vacuum at temperatures ranging from 2300 to 2800℃. The high temperatures help volatilize interstitial impurities. Niobium components can be sintered to near full density at temperatures of 1900-2000℃ in high vacuum (better than 10-7 Torr). The effects of sintering temperature on density and grain size are shown in Fig. 23.16. As the density exceeds 90%, grain boundaries break away from pores resulting in rapid grain growth. Similar densities were achieved for MIM Nb sintered at 1800-2000℃ in vacuum (10-3 to 10-5 Torr), but the resulting oxygen and carbon contents were 0.03 and 0.02 wt%, respectively, and NbC was precipitated at the grain boundaries.
Solution-strengthened Nb and Ta alloys can be processed from prealloyed hydridedehydride powders. Prealloyed Nb-30Hf-9W (C-2009) can be injection molded and vacuum sintered to 94% of the theoretical density at 2250℃. Full density can be achieved with subsequent containerless hot isostatic pressing (HIP) at 1950℃ for 3 h at 200MPa with carbon and oxygen contents of 80 and 230 ppm, respectively, which are below the critical levels that cause brittle behavior. The sintering response of PIM Nb-12Hf-9W-0.1Y (C-129-Y) is poorer, resulting in a maximum density of 91% of theoretical at a sintering temperature of 2350℃ . Prealloyed Ta-9.2W-0.5Hf powders have been HIPed at 1300-1500℃ to densities of 99% of theoretical. Strengths were higher than the melted and rolled alloy, but elongations were lower. Additional pressureless sintering at 2200℃ improved elongation, but decreased strength with little effect on the density.
Fig. 23.16 Plot of density and grain size versus sintering temperature for Nb
Solid-state sintering generally does not produce a fully dense component. For traditional processing of refractory metals, low sintered densities can be overcome by thermomechanical working, but this is not an option for net-shape MIM components. Sintering to <8% porosity is necessary for closed porosity to enable further consolidation by HIP without external canning. Still, HIP is typically limited to temperatures of 2000℃ and pressures of 200MPa, which may not be sufficient for the most difficult to densify refractory metals. Other pressure-assisted consolidation techniques, such as rapid omnidirectional compaction (ROC) and spark plasma sintering (SPS) have been used to densify W, but these methods do not seem practical for net-shape MIM components.
Homogeneous refractory metal alloy powders are required for improved densification and to avoid inclusions from undissolved particles or precipitates. For example, with Re coated particles, a W-5 Re alloy can be completely homogenized in 30min at 2230℃, while homogenization of the same composition produced from a blend of W and Re powders takes up to 60 h. Similarly, mechanical alloying improves the homogenization of a W-25 Re alloy over blending. These methods of adding Re to W can also be used to prepare other solution strengthened alloy powders such as W-Mo, Mo-Re, and Mo-W. Mo alloys such as Mo-0.5Ti, Mo-0.5Ti-0.1Zr (TZM), and Mo-1.2Hf-0.1C (MHC) can be produced by milling elemental powders or their hydrides with Mo and sintering at 1920-1980℃ in hydrogen.
Homogeneous distributions of oxides and carbides, such as ThO2, CeO2, ZrO2, HfO2, Er2O3, La2O3, Y2O3, TiC, TaC, HfC, or ZrC for dispersion strengthening of W and Mo, can be achieved by processing appropriate precursors with the refractory metal precursor. Nitrates, such as La(NO3)3, which can be added as aqueous solutions, are common precursors. Such precursors decompose into oxides that are thermodynamically stable even during hydrogen reduction. Hydrides, such as ZrH2, can be used as precursors along with carbon additions to form in situ carbides. Powder forms of the dispersoids must generally have particle sizes of <1μm to effectively pin grain boundaries. Additive amounts can range from 0.3 to 4 wt%.
Unlike liquid-phase sintered heavy alloys, distortion is not a major concern for refractory metals that are solid-state sintered. Although the high density of W causes shrinkage in the vertical direction to be about 0.5% more than in the horizontal direction, the dimensional precision of MIM W parts is mainly determined by the injection molding process, especially the injection pressure. With optimized molding parameters and a 2-3μmW powder, dimensions can be held to ±0.09% after sintering at 2300℃ to a relative density of 92.9%.
Relative sintered densities of 99% can be achieved for MIM W parts with a 1-2μmW powder by sintering at 2400℃ or higher. MIM W parts sintered at 2400℃ are ductile at 200℃, while those sintered at 2600℃ are ductile at 400℃. Higher strengths can be achieved with 1 wt% TiC additions, while still achieving a relative density of 99% and ductility at 400℃.
Contact: Cindy Wang
Phone: +86 19916725892
Tel: 0512-55128901
Email: [email protected]
Add: No.6 Huxiang Road, Kunshan development Zone, JiangsuShanghai Branch: No. 398 Guiyang Rd, Yangpu District, Shanghai, China