Refractory metals, heavy alloys, and hardmetals are typically fabricated by powder processing methods. Their main constituents are extracted from ores as powders and their high melting temperatures prevent processing by conventional melt-based forming technologies. Metal injection molding (MIM) is an attractive forming technique for these materials since it reduces the need for secondary operations. These secondary operations can prove expensive for refractory metals and heavy alloys owing to both the high cost of material scrap and the difficulty in working or machining them. Hardmetals cannot be machined and must be ground, so near-net shaping is of even greater advantage for complex shapes that cannot be formed by traditional means. Since processing of all these materials usually starts with powders, MIM is not at a material cost disadvantage. In fact, MIM provides a second significant cost advantage in reduced loss and easy recycling of costly material. Thus, MIM of refractory metal and hardmetal components, even large ones that may be more technically challenging, offers a compelling cost benefit.
MIM has been demonstrated for many refractory alloys, including tungsten, tungsten heavy alloys, tungsten-copper, rhenium, molybdenum, molybdenum-copper, niobium-based alloys, and several others. These metals, alloys, and composites possess unique combinations of strength, hardness, ductility, toughness, density, and temperature resistance, and thus they form an important class of materials that have been accepted for MIM. Applications range from ordnance components to electrical and medical electrodes.
In comparison to stainless steel MIM, where the powders are typically spherical, refractory metal, heavy alloy, and hardmetal powders have high surface area to volume ratios and irregularly shaped particles which cause the feedstock formulations to have lower quantities of powders and higher quantities of polymer for a moldable viscosity. This lower solids loading of powder requires the tooling to have greater shrinkage as compared to stainless steel and iron-based MIM. Also, these materials are sintered at a much higher temperature as compared to more conventional stainless steels and iron-based alloys. Thus, the processing is more along the lines of ceramic injection molding, where the feedstock formulations have lower solids loading and the sintering temperatures are higher. The significant difference is that refractory metals, heavy alloys, and hardmetals are typically sintered in hydrogen or vacuum, whereas ceramics are typically sintered in air.
This chapter provides an overview of the application and processing of this important class of metals, alloys, and composites. Applications, alloy additions, powder preparation, sintering, and mechanical properties are discussed.
As their names suggest, refractory metals are typically used in high-temperature applications; heavy alloys are used where high density is desired and hardmetals are used where high wear resistance is required. Heavy alloys and hardmetals are based on W, which is the most common refractory metal. General properties of W and some other refractory metals are given in Table 23.1.
Table 23.1 General properties of refractory metals
Property | Mo | Nb | Re | Ta | W |
Melting point (°C) Density (g/cm3) Thermal expansion (ppm/°C) Thermal conductivity (W/m per °C) Electrical resistivity (μohm-cm) Tensile strength (20°C, MPa) Modulus (20°C, GPa) Crystal structure (20°C) | 2617 10.2 4.8 142 5.4 1030 330 BBC | 2468 8.6 7.3 52 14.4 550 130 BBC | 3180 21.0 6.2 71 18.5 1380 450 HCP | 2996 16.6 6.3 54 13.1 340 190 BBC | 3410 19.3 4.5 166 5.3 2070 410 BBC |
Tungsten's melting temperature of 3410℃ is the highest of any metal. It has high strength, high density, high electrical and thermal conductivity, and a low-thermal expansion coefficient. These properties make W useful for filaments, electrodes, electrical contacts, heat sinks, thermocouples, ion implant components, furnace components, hot forming dies, shaped charge liners, and specialty springs and fasteners. Plasma facing components, such as modular cooling fingers, are an emerging application for MIM W.
Alloying elements that are soluble in W can improve its properties through solution strengthening. Rhenium is especially effective at increasing the high temperature strength of W and improves its room temperature ductility through the "rhenium effect", which is also observed for Mo. Common alloying levels of Re range from 3 to 26 wt% for W and up to 51 wt% for Mo.
Tungsten can be dispersion strengthened with oxides that pin grain boundaries for improved high-temperature strength and creep resistance. Common examples include additions of up to 2wt% ThO2, La2O3, Ce2O3, or Y2O3. These oxide additions limit grain growth to provide the necessary service life in applications that receive cyclic heating, such as electrodes and furnace components. Carbides generally have lower thermodynamic stability than oxides but can also be used to improve mechanical properties. HfC has the highest melting temperature and greatest thermodynamic stability and does not adversely affect the room temperature ductility of W.
Alloys of tungsten and combinations of Ni, Fe, Co, and Cu are called heavy alloys. They consist of W grains in a metal matrix and are used for shielding or collimating energetic X-ray and γ-radiation, kinetic energy penetrators, electrodes, and various types of weights. Smaller heavy alloy components, such as cell phone vibrator weights, medical electrodes, and small inertia products are routinely injection molded. An example of a MIM heavy alloy counterweight is given in Fig. 23.1.

Fig. 23.1 Tungsten heavy alloy disk drive counterweight
Tungsten can be combined with carbon to form tungsten carbide, which has very high hardness. Tungsten carbide can be combined with metals, such as Co, Ni, and/or Cr, to produce hardmetals, also known as cemented carbides. These composites consist of WC particles "cemented" together in a ductile metal matrix and have unique combinations of hardness and toughness, which make them widely used for tools for high-speed machining, components in earth-boring bits, road planing, and wear parts such as dies, anvils, and rolls. Some example WC-Co components that have been molded are percussive mining tips, watchcase rings, and other complex shaped tools for wear and cutting applications.
Molybdenum is chemically similar to W, but its lower density is an advantage for applications where weight is important. A common Mo alloy is TZM, which contains small additions of Ti, Zr, and C to significantly increase high temperature strength. Molybdenum is also commonly oxide-dispersion strengthened with La2O3 additions of 0.3-0.7wt% to greatly increase its creep resistance. Molybdenum, TZM and Mo-La2O3 are used for electrodes, ion etching grids, thermocouples, furnace components, hot forming dies, injection molding tooling, X-ray targets, crucibles for sapphire crystal growth, electrical contacts, shaped charge liners, heat sinks, and specialty springs and fasteners. Mo-Re alloys are used for stents, thermocouples, and rocket nozzles.
Rhenium has the second highest melting temperature of any metal. Unlike W, Re has a ductile-to-brittle transition well below room temperature. Rhenium has the highest tensile and creep rupture strength of the refractory metals and is virtually inert to thermal shock. Its wear resistance is second only to Os, and it also has the second highest strain hardening coefficient of the refractory metals. The main disadvantages of Re are its scarcity and high oxidation rate. Owing to its high cost and the difficulty in shaping it, commercial fabrication of Re and its alloys is limited. Most applications are military, dealing with rocket systems that require high-temperature strength and high ductility.
Tantalum is biocompatible and approved for use in long-term surgical implants as specified by ASTM F560. Its melting temperature and density are lower than those of W, but much higher than those of more commonly used metals. It is soft, ductile, and easy to machine. The largest application for Ta is in electrolytic capacitors owing to the dielectric properties of its oxide (Ta2O5), but it is also used for surgical implants in the forms of wire, foils, sheets, clips, staples, and meshes. It has also been considered as an implantable counterweight for some medical devices. Tantalum can be solution strengthened by additions of W and Hf for improved properties at temperatures over 1430℃.
Niobium is chemically very similar to Ta. It has a lower melting temperature and at 8.57 g/cm3 , Nb has the lowest density of all the refractory metals and is thus a leading candidate for applications where the strength to weight ratio is a critical requirement, including some specialty springs and fasteners. Like Ta, Nb can be strengthened with W and Hf additions. Most Nb and Ta alloys are relatively low-strength alloys produced by electron beam melting, high-temperature extrusion, and forging. MIM offers potential advantages for net-shape manufacturing of higher strength alloys, but high purities and uniform microstructures are required to attain the desired properties.
Feedstocks for refractory metals, heavy alloys, and hardmetals are similar in powder/ polymer ratio to ceramic injection molding and similar in polymer formulation to other metal systems. Typically, the solids loading is in the 50-60 vol% range and the polymers can be of the wax-polymer, water-soluble, or catalytic variety. These solids loadings translate into average tooling scale-up factors of 1.18-1.26, assuming that the part is sintered to full density. The powder content in the feedstock is limited by the high surface area of finer particles (1-4μm) as compared to typical gasatomized metals (5-20μm), which have a powder loading of up to 67 vol%.
Owing to their high melting temperatures, refractory metal powders are generally produced by thermally processing chemical precursors. The nature of the chemical precursor and the temperature, time, and atmosphere of the thermal processing cycle determine the powder characteristics, which affect molding and sintering behavior.
Tungsten and Mo powders are typically produced by hydrogen-reduction of oxides. Tungsten particle sizes are typically 3-5μm but can range from about 0.1 to 50μm depending on the time, temperature, hydrogen flow rate, dew point, and depth of the powder bed. Molybdenum particle sizes can be adjusted over a narrow range of 1-6μm by the same variables that control W particle size. Composite powders can be produced by co-reducing tungsten and molybdenum oxides with other metal oxide powders. Oxides and carbides for dispersion strengthening or their precursors are also often added prior to hydrogen reduction of W or Mo powders.
Table 23.2 Characteristics of example refractory metal and hardmetal powders
Refractory metal | W | Mo | Re | Ta | Nb | Wc |
Chemistry | ||||||
O (wt.%) C (wt.%) H (wt.%) FSSS | 0.120 0.0057 - 1.25 | 0.606 0.0017 – 2.2 | 0.090 <0.001 0.0056 3.5 | 0.178 0.0009 0.166 2.4 | 1.80 0.0068 0.4000 2.9 | 0.091 6.14 - 1.32 |
Particle size distribution | ||||||
D10 (μm) D50 (μm) D90 (μm) | 0.88 2.7 9.8 | 2.8 6.2 7.0 | 7.5 17.5 32.8 | 1.8 5.0 7.7 | 3.7 7.4 12.4 | 0.9 1.7 3.2 |
BET | ||||||
Specific surface area (m2/g) | 0.65 | 0.48 | - | 0.39 | - | 1.00 |
Particle size (μm) | 0.49 | 1.23 | - | 0.90 | - | 0.39 |
Pycnometer density (g/cm3) | 19.0 | 10.1 | 20.7 | 16.5 | 8.4 | 15.5 |
Apparent density(g/cm3) | 3.6 | 1.9 | 1.6 | 4.7 | 2.1 | 2.7 |
% of pycnometer | 19% | 18% | 8% | 28% | 25% | 17% |
Tap density (g/cm3) | 4.9 | 2.7 | 2.9 | 6.0 | 3.0 | - |
% of pycnometer | 26% | 26% | 14% | 36% | 36% | - |
After reduction, W and Mo powders must be deagglomerated, typically by milling, to produce suitable MIM feedstock. Otherwise the solids loading will be too low at a moldable viscosity. They also often need to be combined with alloying additions, such as Ni and Fe in the case of heavy alloys. The deagglomerated W or Mo powder can be dry mixed with other metal powders using a double-cone, v-cone, or Turbula mixer, or the reduced W or Mo powder can be co-milled or coated with the alloying additions.
Tungsten carbide powders are produced by carburizing W powders. The characteristics, especially the particle size, of the WC powder depend primarily on the starting W particle size and the carburization temperature and time. Chemistry control is critical. Carbon contents must be held constant near the stoichiometric value of 6.13wt%. Small amounts of oxides of vanadium and/or chromium can be added prior to carburization to control the grain size through later processing steps. VC and Cr3C2 as well as TiC, TaC, and NbC, can be added when the WC powder is milled with the metal matrix.
The conditions for milling the WC powder with the Co powder and any other additives to make hardmetal powders are key processing parameters. Two common milling techniques are ball milling and attritor milling. Both processes homogenize the mixture and can result in particle size reduction. After milling, the powders are usually spraydried. Most commercial spray-dried graded hardmetal powders contain a binder, such as wax or polyethylene glycol, which is added during milling to hold together free-flowing, spray-dried agglomerates and later to provide sufficient strength in pressed parts. Such powders can be used for MIM if the binder in the spray-dried powder is compatible with the MIM binder. Otherwise, a custom lot without a binder must be produced or the powder must go through a debinding cycle prior to compounding the feedstock.
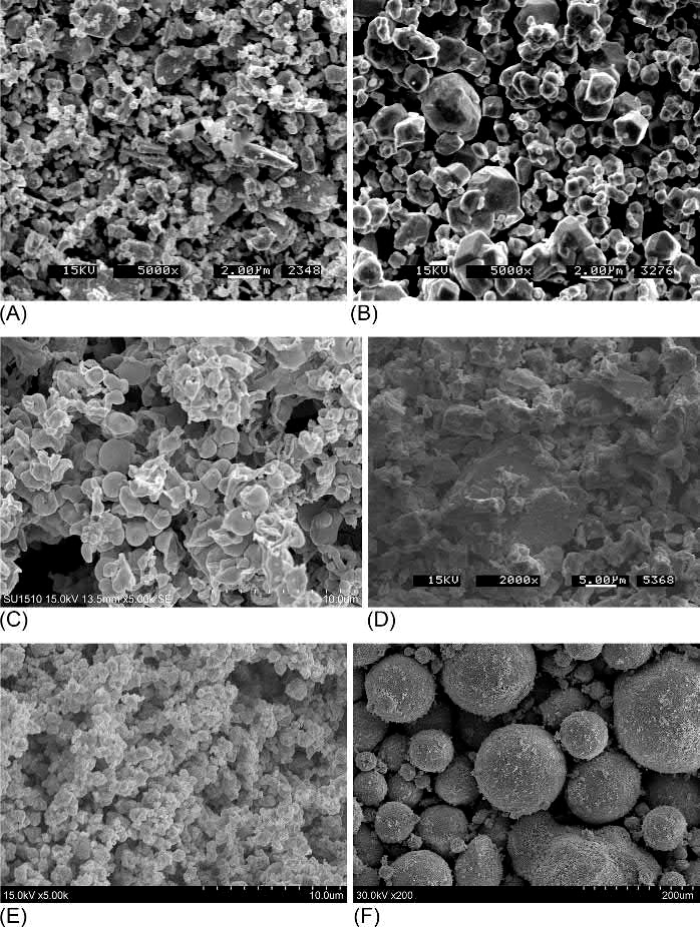
Fig. 23.2 SEMs of (A) 2.7μmW powder. (B) 6.2μm Mo powder. (C) 17.5μm Re powder. (D) 7.4μm Nb powder. (E) 1.7μm WC powder. (F) Spray-dried WC-10% Co powder
Hardmetal powders can also be produced from recycled scrap material via a zincreclaim process. These "reclaimed" powders often provide more predictable molding behavior since they have less surface area than WC powders made directly from the carburization of W.
Rhenium powders are produced by a two-stage hydrogen reduction process in which ammonium perrhenate is first reduced to an oxide before final reduction to Re metal. Different temperatures and hydrogen flow rates are used for the two stages. Typical particle sizes are 1-3μm . Typical Re powders are agglomerated and have poor packing characteristics with apparent densities of 1.2-1.8 g/cm3 . Coarse spherical Re powders are also available for MIM; however, their sintering response is reduced owing to their lower surface area.
Production of Ta powders starts by reducing potassium tantalum fluoride with sodium, while production of Nb powders starts by reducing niobium oxide with Al. The resulting products are subsequently melted with an electron beam, hydrided, crushed, dehydrided, and milled to produce angular particles. Alloying elements can be included in the melt. Tantalum and Ta alloy particle sizes range from 3μm to 6μm, while Nb and Nb alloy particle sizes range from 10μm to 15μm (Wolfe et al., 2015). Further milling can be used to round the particles if desired.
The characteristics of some example commercial powders are summarized in Table 23.2. The most commonly used refractory metal and refractory metal carbide powders have particle sizes ranging from about 1μm to a few μm. Particle sizes below 1μm are used in specialty applications but increase contamination concerns owing to their high surface area. Particles coarser than a few μm can likewise be used in special situations, but their poor sintering behavior makes them less useful. Oxygen is the main impurity in refractory metal powders and can significantly lower their measured densities from their theoretical values. The agglomerated nature of the powders is indicated by their low apparent and tap densities. Their irregular particle morphologies can be seen in the scanning electron micrographs (SEMs) in Fig. 23.2, which also includes a spray-dried WC-Co powder.
The small, irregular particles of refractory metal and hardmetal powders result in relatively low solids loadings. As examples, Fig. 23.3 shows a plot of the torque required to mix W-Cu and Mo powders of various particle sizes at different solids loadings with a paraffin wax-polypropylene binder. From this plot the optimal solids loading for W-15Cu decreases from 58 vol% for the 0.23μmW powder to 52.5 vol% for the 0.11μmW powder. Similarly, the optimal solids loading decreases from 62 vol% for the 1.52μm Mo powder to 58 vol% for the 0.47μm Mo powder.
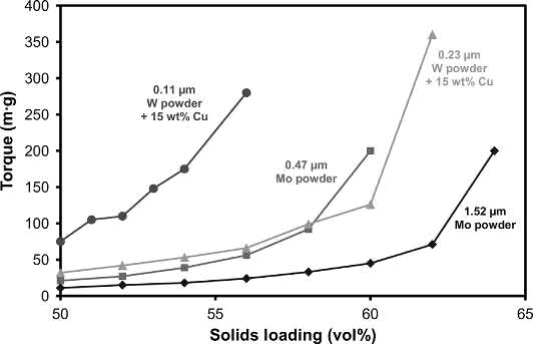
Fig. 23.3 Effect of solids loading on mixing torque for W-Cu and Mo powders with different BET particle sizes
Because of the dispersion difficulties with fine-grained powders, high shear rate continuous mixers are preferred for compounding. Inhomogeneous binder distribution leads to higher viscosity in the feedstock, since a high concentration of binder in one area results in a low concentration elsewhere. The areas of low concentration of binder will increase viscosity due to interparticle friction of the under lubricated powder. Poor dispersion of binders can cause sintered defects in WC-Co MIM parts. Areas of high concentration of organic binder, once removed in the debinding process, leave behind pores that can be too large to eliminate in vacuum sintering. Pressure assisted sintering can eliminate the pores in the part interior, but not on or near the surface where the gas pressure enters the pores. Also, the interior pores can be filled with cobalt by pressure assisted sintering, which will negatively affect mechanical properties. Surface pores, if not removed by grinding, will also negatively affect mechanical properties.
The abrasive nature of refractory metal and especially hardmetal powders results in greater wear of mixer components, which increases processing costs. The contamination of heavy alloys and hardmetals from wear is of limited concern since they usually contain at least small amounts of iron, but the processing and properties of refractory metals are more sensitive to contamination, as discussed in Section 23.5.2.
Examples of several heavy alloy, refractory metal, and hardmetal feedstocks are summarized in Table 23.3. Wax-polymer binders perform adequately for these materials. Solids loadings as low as 46 vol% have been reported for water-based gelling systems, wax-based low-pressure systems, and with nanograined powders. Solids loadings as high as 65 vol% can be achieved with jet milled W powder. For heavy alloy feedstock, deagglomerating the W powder before mixing increases the maximum solids by about 3%, but the agglomerates have no effect on the microstructure or mechanical properties of sintered parts. For WC-Co hardmetals, the optimal surfactants are fatty acids with long carbon chain lengths, such as octadecanoic (stearic) acid. In general, molding and debinding are similar to other MIM feedstocks.
The addition of lower melting temperature transition metals such as Ni, Fe, Co, and Cu to refractory metals, especiallyW, can producetwo-phase alloys with unique properties that can be processed by liquid-phase sintering at lower temperatures than commercially pure refractory metals. The most common heavy alloys contain W with Ni-Fe or Ni-Cu matrices, although other transition metals such as Co, Cr, Mo, and Mn are sometimes added or substituted to improve properties or lower sintering temperatures. Limited solubility ofthetransition metal additions in the refractory metal and high solubility of the refractory metal in the transition metal benefit processing, but the tradeoff is decreased high-temperature performance. Still, such matrix formers enable other unique properties of refractory metals, such as high density or low thermal expansion coefficient, to be more easily utilized.
Table 23.3 Examples of heavy alloy, refractory metal, and hardmetal feedstocks
Binder | Composition | Solids loading vol% | Mixing technique |
Polyethylene wax | W-4.9Ni2.1Fe | 49 | Double planetary |
Polyethylene Polystyrene Oil | W-4Ni-1Fe | 50 | Double planetary |
Paraffin wax Polypropylene | W-2.1Ni0.9Fe | 60 | Twinscrew |
50% microcrystalline petroleum wax 29% montan ester wax 21% synthetic hydrocarbon wax | WC-6Co | 65 | Paste mixer with trifoil blade |
54%–65% paraffin wax | WC-11Co | 56.5 | Twinscrew |
30%–36% polypropylene 5% octadecanoic acid | WC-6Co WC-15Co | 59 55 | |
65% paraffin wax 15% low-density polyethylene 15% vegetable oil | WC-5TiC10Co | 57 | Roller mixer |
Paraffin wax Polypropylene Polyethylene Stearic acid | Nb | 57 | Twinscrew |
Polybutene | W | 55 | Paste mixer with impeller |
Polyethylene wax | WC | 55 | |
Polyethylene glycol Stearic acid | WC-10Co | 56.8 | |
65% paraffin wax 10% high-density polyethylene 10% polypropylene 5% dioctyl phthalate 5% ethylene propylene diene monomer 5% stearic acid 4.3% petroleum jelly | WC-8Co | 62 | Self-made mixing device |
43.3% poly(ethylene-co- (alpha-octene)) 39% paraffin wax 5.6% FisherTropsch wax 7.7% stearic acid | WC-13Co | 55.3 | Sigma blade |
Matrix materials increase the ductility of the alloy and contain W in solid solution.In the case of a pure Ni addition, up to 40 wt% W can remain in the matrix after cooling to room temperature, however formation of the Ni4W intermetallic phase results in poor mechanical properties. Iron additions to W-Ni reduce both the solubility of W in the matrix and the tendency to form intermetallics. The Ni:Fe ratio should generally be in the range of 1.5-4. Intermetallic formation with Ni:Fe ratios up to 15 can be reduced by resolutionizing and quenching. Ni:Fe ratios below 1.2 result in the formation of Fe7W6 intermetallics that cannot be resolutionized in a subsequent heat treatment.
Copper additions to W-Ni reduce the solid solution content of W in the matrix but are less effective at suppressing Ni4W formation. Tungsten can also precipitate from the matrix phase during cooling, resulting in inclusions that lower strength and ductility. Rapid cooling of W-Ni-Cu alloys enables greater supersaturation of the matrix and improved mechanical properties, but the ductility is less than that of W-Ni-Fe alloys. The main use for W-Ni-Cu alloys over W-Ni-Fe alloys is for applications that require a nonmagnetic alloy. Both types of heavy alloys can be further tailored to specific applications by adding other transition metals to adjust static and/or dynamic properties.
Contact: Cindy Wang
Phone: +86 19916725892
Tel: 0512-55128901
Email: [email protected]
Add: No.6 Huxiang Road, Kunshan development Zone, JiangsuShanghai Branch: No. 398 Guiyang Rd, Yangpu District, Shanghai, China