The majority of hardmetals are based on combinations of tungsten carbide and cobalt.Cobalt is most commonly used in contents of 3-25 wt%, but Ni and Cr are used in applications that require enhanced corrosion resistance. The metal binder can be further modified through additional alloying. For example, Ru additions to WC-Co hardmetals significantly increase hardness without decreasing its toughness. Lower binder contents also increase hardness, but at the expense of toughness.
Hardmetal compositions can be divided into three basic types: straight grades, micrograin grades, and alloyed grades. Straight grades are primarily WC in a Co binder but may contain small amounts of grain growth inhibitors. Micrograin grades consist of WC in a Co binder with several tenths of a percent of VC and/or Cr3C2 to achieve a grain size of <1μm. Alloyed grades consist of WC in a Co binder with additions of Ti, Ta, and Nb, which form separate carbide grains that have a more rounded morphology. These are commonly referred to as cubic or solid solution carbides.
Straight grades for metalworking generally contain 3-12 wt% Co. The WC grain size usually ranges from 1 to 8μm. As the grain size decreases, hardness and strength increase, but toughness decreases. Components for rock and earth drilling tools are produced from straight grades containing 6-16 wt% Co with grain sizes ranging from 1.5 to 10μm or larger. Straight grades for dies and punches have a medium grain size and Co contents ranging from 16 to 30 wt%.
Micrograin grades generally contain 6-15 wt% Co. The VC and/or Cr3C2 additions control grain growth during LPS, resulting in a final grain size of <1μm. The fine grain size gives very high hardness and strength, which is especially useful for cutting tools for soft workpiece materials because they can be highly polished and can hold an extremely sharp cutting edge. They can also be used for machining Ni-base superalloys because of their ability to withstand temperatures up to 1200℃C. Micrograin grades are also desired for rotating tools that generate shear stresses, such as drills.
Alloyed grades are primarily used for cutting steel and typically contain 5-10wt% Co. The grain size ranges from 0.8 to 2μm. TiC additions range from 4 to 25wt% and reduce the tendency of WC to diffuse into steel chip surfaces. TaC and NbC additions range from 0wt% to 25wt% and improve strength, cratering resistance, and thermal shock resistance. These cubic carbide additions also increase the hot hardness, which helps to avoid thermal deformation in applications where high temperatures are created at the cutting edge. Alloyed grades are also used for nonmetal working applications, primarily as wear parts. Typical grain sizes range from 1.2 to 2μm with Co contents ranging from 7 to 10wt%. Wear grades for applications requiring increased corrosion resistance and higher hardness are manufactured with additions of Ni and Cr.
Practically all WC-Co hardmetals are processed by LPS, which was described in Section 23.4.1. Both W and C are soluble in Co, and the ternary system forms a eutectic at about 1280℃C. Densification occurs rapidly when the eutectic liquid forms. The liquid phase enables achievement of densities above 99% of theoretical even with coarse WC powders and at low Co contents. Hardmetals need to be sintered to full density, since very small amounts of porosity can make them brittle. Hardmetals can be solidstate sintered to high densities, but LPS is generally required to develop the optimal microstructure.
Grain growth is controlled by the reaction at the interface of WC grains rather than by diffusion in the liquid phase, as in heavy alloys. Grain-growth rates for hardmetals are much lower than for heavy alloys, allowing for much smaller grain sizes. During isothermal sintering, the grain size increases with the square root of time. The rate of the interfacial reaction, and thus the rate of WC grain growth, can be controlled by small additions (<1 wt%) of other transition metal carbides, including VC, Cr3C2, NbC, TaC, TiC, and Zr/HfC, which are listed in decreasing degree of effectiveness.
The effect of VC additions on the grain size of WC-7Co with a 0.050μm starting particle size is shown in Fig. 23.17. The undoped WC-7Co powder must be sintered below 1400℃C to maintain a submicron particle size, while VC doping is able to preserve a submicron grain size even at a sintering temperature of 1480℃C. Since grain growthislimited in doped hardmetals, the final grain size is generally related to the starting particle size.
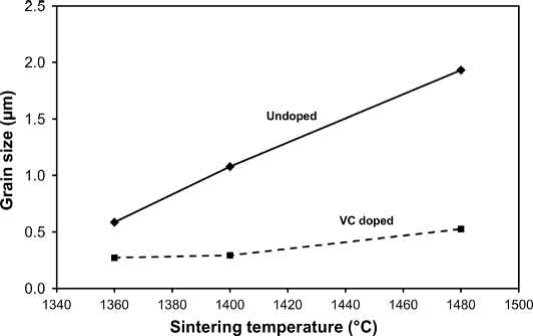
Fig. 23.17 Effect of sintering temperature on the grain size of doped and undoped WC-7Co with a starting WC particle size of 0.05μm
Abnormal grain growth, in which a few large grains grow several times larger than the average grain size, can occur during LPS of WC-Co. In this case, a powder with a smaller average particle size can result in a larger sintered grain size than a powder with a larger average particle size. Abnormal grain growth can be avoided by using a powder with a narrow particle size distribution. Lower sintering temperatures and the use of grain growth inhibitors also reduce the occurrence of abnormal grain growth.
Hardmetals are typically sintered in vacuum batch furnaces at 1400-1450℃C, but temperatures may range from 1350 to 1600℃C, depending on the Co content and the desired microstructure. The furnace hot zone is generally constructed from graphite and MIM hardmetal components are set on graphite trays coated with graphite paint. The vacuum level during sintering is usually about 0.1 Pa (10-3 Torr), but special furnaces capable of backfilling the furnace with argon to a pressure of at least 3MPa while the matrix is still molten to ensure the parts are fully densified. The use of pressure assisted sintering has become increasingly common to virtually eliminate scrap due to residual porosity. Conventionally vacuum-sintered components can be HIPed to full density in a separate operation, but at additional cost. They can also be used in some applications without HIPing, but generally require higher sintering temperatures that can coarsen the microstructure. A smaller WC particle size and/or the addition of grain growth inhibitors can help maintain a fine grain size.
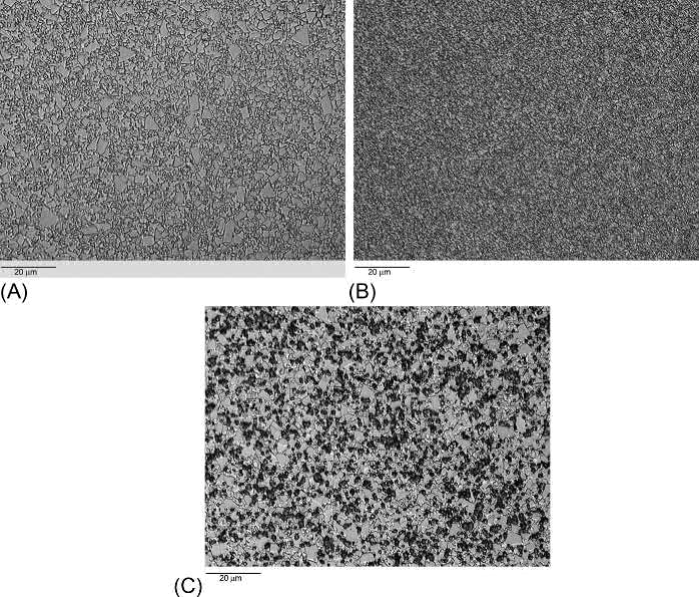
Fig. 23.18 Sintered microstructures of (A) A straight grade. (B) A micrograin grade. (C) An alloyed grade showing cubic carbides
Specialized sintering cycles and adjustment of the powder chemistry can allow for enrichment or depletion of the Co at the outermost 20-30μm of the sintered component. An enriched layer can give the component the performance of a tougher grade with the deformation resistance of the lower binder content below the surface. A depleted layer can provide a better surface for deposition of wear-resistant coatings, such as TiC, TiN, TiCN, TiAlN, or Al2O3.
Example microstructures of a straight grade, a micrograin grade, and an alloyed grade are shown in Fig. 23.18. The straight grade contains relatively large grains in comparison to the micrograin grade, but they are still much smaller than seen in liquid-phase sintered heavy alloys. The fine, irregular grains make WC-Co much less susceptible to gravity-induced distortion than heavy alloys. The dark, blocky areas in the alloyed grade are cubic carbides.
The injection molding process requires careful control of trapped air, weld lines, and flash. Since carbides are much more sensitive to microstructural defects than metals, any large pores in the green body created by jetting or poor weld lines will result in unacceptable microstructures. Control of flash is a challenge owing to the small particle size of the powders, allowing feedstock to enter much smaller gaps in the tooling than would a larger steel powder. If cutting inserts are to be molded, flashing at the cutting edge will limit the attainable cutting-edge radius, or hone size, since green removal of the flash will result in a chamfered edge. Postsinter honing can round this chamfer to a radius, but the hone size will exceed the thickness of the original flashing. Adding to this difficulty is the high abrasive wear imparted by the carbide feedstock to areas of the tooling that flash, which only increases the gap and limits tool life. However, with a proper fitting mold, cutting inserts can be produced nearly flash free, requiring little or no post processing after sintering.
The avoidance of flash has led to development of low-pressure injection molding of carbides, allowing for longer tool life while using lower cost tool materials and molding equipment. The binder and solids loading are customized to achieve a low-viscosity slurry that can be injected at lower pressures. Low-pressure injection molding may lead to difficulty in packing thick sections, resulting in sinks or voids. This can be partially compensated with a larger gate and increased mold temperature to extend the packing time. This also requires the gate to be located at or near the thickest cross-section. Injection molding of micro-sized parts is also possible, provided the rheology is adjusted to allow filling of micro features under normal injection pressures. This can be achieved by a reduction in the powder loading and proper selection of binders.
Cracks, sinks, and voids often occur in ceramic and carbide injection molded parts due to the stresses in the green compact. As thermoplastics cool, they contract by pressure-volume-temperature (PVT) response. These shrinkage stresses can cause cracks in the case of a restriction of shrinkage by the tooling. An example is an inner diameter created by a tool core in a thick section. The tooling restricts shrinkage of the compact, creating tensile stresses in the inner diameter. If poor weld lines or other weak points exist in the compact, cracks may initiate there from these stresses. Injection molded components with thick sections typically freeze at the gate before freezing occurs in the thickest sections. The resulting shrinkage of the material creates shrinkage stress, which can result in voids or sinks in the compact. The solutions for voids and sinks may include increasing the gate size, moving the gate to a thicker section of the part, use of a heated sprue, increasing die temperature to extend the time before gate freeze, or increasing hold pressure before gate freeze. Too much pressure, however, leads to stress variations in the part that may cause cracks. Incorporation of an amorphous polymer or low melting temperature polymer can reduce the overall shrinkage. In an amorphous polymer, there is no glass transition, which eliminates a portion of the shrinkage that would be observed in a crystalline polymer. Use of a lower melting temperature polymer in a feedstock can reduce the required molding temperature, which can in return reduce the total expansion of the system, and thus reduce the shrinkage upon cooling. Use of a flexible polymer can allow for stresses to be accommodated without causing cracks.
All-thermal debinding of wax-based systems can be done successfully but requires long thermal cycles. Deformation of the body can occur due to gravitational forces, especially in thick cross-sections. A powder bed of alumina and graphite may be used to assist in wicking the binders out and avoiding distortion. Others have also demonstrated all-thermal debinding of wax-polymer systems, though this may result in a decrease in dimensional precision.
Thermal debinding of carbides is similar to tool steels, requiring close attention to carbon control. Hydrogen may be used but can cause decarburization. Decarburization may also be caused by reaction of carbon with oxides in the powder, and carburization is possible if binder removal is incomplete, which is most likely to occur with inert atmospheres.
Debinding in nitrogen/hydrogen blends and vacuum debinding can prevent decarburization. Thermal debinding of the polymer fraction of the binder system in a 20%-50% H2/balance N2 atmosphere at a maximum temperature of 500℃C resulted in no change in carbon compared to the starting powder. A percentage of H2 higher than 50% resulted in decarburization, while a percentage of H2 lower than 20% resulted in residual carbon remaining after sintering. For all-thermal debinding of a wax-polymer system to a maximum temperature of 600℃C, 100% N2 resulted in carburization, 100% H2 resulted in decarburization, and 75% H2/balance N2 resulted in balanced carbon. When the same system underwent a two-stage debinding process with solvent extraction first, followed by thermal debinding under a faster cycle, equal balanced carbon was obtained with 75% H2/balance N2 and 100% H2. This was attributed to the short hold time at the maximum debinding temperature of 600C. The optimal H2/N2 ratio may depend on the binder composition, gas flow rate, part cross section, and/or furnace load.
The carbon content of hardmetals must be carefully controlled to achieve optimal properties. High carbon levels will cause free carbon to precipitate out in the microstructure. Low carbon levels will result in the formation of embrittling double carbides, such as Co3W3C or Co6W6C, called η phase. The carbon window depends on the Co content. The higher the Co content, the wider the limit on carbon levels. Hardmetals with matrix alloys based on metals other than Co or Ni usually require even tighter carbon control.
Many factors affect carbon levels including the chemistry of the starting powder, binder composition, component size, debinding method, furnace construction, furnace load, sintering substrate, sintering atmosphere, heating rate, sintering time, and temperature. The carbon content of sintered components is usually determined nondestructively by measuring their magnetic saturation. If the hardmetal is undercarburized, the Co matrix will dissolve more W, lowering its magnetic saturation. A magnetic saturation below 78% that of the equivalent carbon-saturated alloy indicates the occurrence of η phase. Precipitated carbon may occur at readings of 100% magnetic saturation, at which point the magnetic saturation stops increasing with carbon content.
In addition to carbon control, grain size is also critical to many hardmetal applications. The grain size can be directly related to the hardness but can be characterized nondestructively and more precisely by measuring the magnetic coercivity, which is related to the interfacial area between the WC grains and Co matrix. The interface restricts movement of magnetic domain walls within the ferromagnetic Co matrix, so a smaller grain size requires a stronger magnetic field to restore zero magnetization to a magnetically saturated hardmetal component.
Although WC-Co components have a low tendency to distort from their own
weight during sintering, they are susceptible to distortion from temperature and carbon gradients in the furnace. Consistent furnace loading is important. Dimensional precision of ±0.2% has been reported. Hardmetals are very sensitive to contamination and should only be sintered in dedicated furnaces.
Sintered hardmetal parts occasionally come out of the furnace coated with a thin layer of Co. This cobalt-capping phenomenon can be beneficial if the parts are to be brazed, but in most cases, it must be ground off. Cobalt-capping can appear to occur randomly from part to part and from furnace run to furnace run. It is related to the carbon activity of the residual gas, which can vary from one location to another in the furnace and on different surfaces of a single product. Cooling in both a nondecarburizing atmosphere and in a decarburizing atmosphere have been recommended to avoid cobalt-capping. Some grades utilize special sintering methods to intentionally drive Co to the surface to increase the toughness of a tool's cutting edge.
In almost all cases, the sintered parts undergo postsintering operations. The minimum operation to a cutting tool is honing of the cutting edge, which is critical to its performance. Many geometries of cutting tools require grinding after sintering. For some tools, the top and bottom of the part will be ground. Others require the periphery to be ground with or without honing of the cutting edge. In many cases, the finished part is coated. The coating provides lubricity and increased hardness. It also provides a diffusion barrier to keep the hardmetal from oxidizing when exposed to the high temperatures encountered during machining.
Contact: Cindy Wang
Phone: +86 19916725892
Tel: 0512-55128901
Email: [email protected]
Add: No.6 Huxiang Road, Kunshan development Zone, JiangsuShanghai Branch: No. 398 Guiyang Rd, Yangpu District, Shanghai, China