The influence of residual carbon may vary markedly depending on the system.A description of the most important systems will therefore be provided in this section. The sintering temperature and carbon content are the most important process variables in tool steels since they dictate the volume fraction of the liquid phase. In general, higher fractions of residual carbon help the densification process, thereby allowing for better control of the process and an increase in the final hardness. In contrast, it is important to achieve a close control of the carbon in cemented carbide systems to avoid carburization or production of the brittle η phase. In other systems, such as stainless steels, magnets (Fe-Si, Fe-Ni), or titanium alloys, the carbon content should be kept to a minimum as such contamination could lead to undesired mechanical, electrical, and magnetic properties. The most important aspects of each system are carefully described in this section.
HSSs have unique physical and mechanical properties that make them good candidates for the production of parts with an optimal combination of high strength, wear resistance, toughness, and hardness. Their production by powder metallurgy techniques results in parts with a uniform distribution of carbides and therefore isotropic mechanical properties. The major disadvantage of this production method for HSS is its moderate sensitivity to sintering parameters such as temperature and atmosphere. Moreover, the optimal conditions are determined by the composition of the HSS, with the carbon content having a particularly pronounced influence on the microstructure evolution and sintering temperature. The initial studies of Shepard et al. showed that the carbon and oxygen contents of the starting material undergo major changes during the sintering process.
The critical dependency of the properties of these steels on carbon content led to the development of a technique to compensate for the loss of carbon during manufacture.The most reliable way of obtaining good results was found to involve blending elemental carbon (graphite) with the metal as it was demonstrated that this process can not only alter the composition but also enhance the sintering kinetics. Finally, it proved possible to explain the sintering mechanism that governs these results. The sintering of these materials takes place by a supersolidus liquid-phase sintering (SLPS) process that allows a near full density to be achieved. The important difference between SLPS and traditional liquid-phase sintering (LPS) is that SLPS produces liquid at the grain boundaries, interparticle boundaries, and inside the grains, whereas LPS produces liquid only at the interparticle boundaries. The temperature and carbon content are the most important variables as they dictate the volume fraction of liquid phase that appears during the densification process.
The high mechanical properties and uniform microstructures obtained by PM, and the possibility to easily achieve near full density by accurately controlling the composition, temperature, and atmosphere, make HSSs good candidates for production by MIM. Moreover, the processing of HSSs by injection molding to obtain near net shape parts avoids costly machining operations. The main challenge in PIM processing of such materials therefore lies in achieving an accurate control of the carbon content. Control of these parameters by controlling the atmosphere is very difficult as the atmospheric composition, CO/CO2 ratio, and the dew point must be controlled and measured accurately throughout the sintering process.
The first method for maintaining an accurate carbon control is to program a presintering step after the initial debinding under hydrogen atmosphere. The temperature of this presintering process must be such that the hydrogen will only attack the free carbon coming from the binder, whilst leaving the combined carbon unchanged, otherwise the parts suffer a heavy loss of carbon which results in a low density and poor mechanical properties. After presintering, the part could be sintered under high vacuum or hydrogen to achieve full densification at temperatures like those used in conventional PM, for example, near to 1240℃ for M2 HSS. This method avoids the distortion that could arise from a nonhomogeneous distribution of admixed carbon. The resulting samples contain amounts of carbon like those of the starting powder (0.8% of carbon in the case of M2, the most common HSS studied because of its industrially interesting applications). If presintering is performed under nitrogen at 700℃, the parts retain only a very small amount of residual carbon (1.02%). This, together with the smaller particle size used in PIM, explains why it is possible to sinter the parts at lower critical temperatures of around 1210℃. The sintering window remains very narrow, at<10℃, in all cases as it is shown in Fig. 14.11.
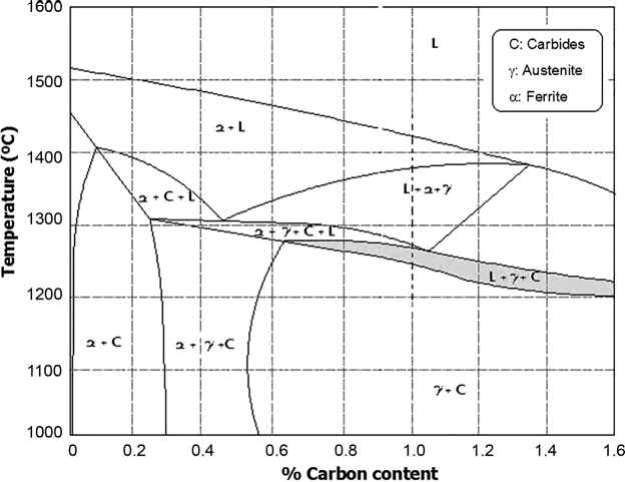
Fig. 14.11 Pseudobinary M2-phase diagram.
The debinding process inherent in MIM could allow a further possibility suggested by several researchers, although one which has apparently been relatively unsuccessful to date, viz., to vary the residual carbon coming from the debinding process. Some of the first researchers to explore this option used a modified MIM process based on a thermosetting binder and M2 as HSS powder. These authors decided to increase the carbon content to prevent carbon loss and found that incomplete debinding led to a notable reduction of the sintering temperature, with a very wide sintering window, for samples containing 3% of residual carbon. This behavior is a consequence of the residual carbon produced upon binder degradation. This expansion of the sintering window allows a microstructural study of the carbide evolution that occurs during the sintering process to be performed, thus giving some idea of the complex sintering process that takes place in this system. The estimation of mechanical properties was made by hardness measurements and the results agree with the values found in similar systems with lower carbon content. The microstructures suggest changes in other properties that have not been evaluated but the observed changes in the sintering temperature are undoubtedly interesting.
The use of incomplete debinding processes by modifying the maximum debinding temperature, as in Fig. 14.12 , permits study of the behavior of steels with different carbon contents in the range between the carbon content of the initial powder and 3 wt%. In industry, the accurate control of incomplete debinding could be difficult from part to part. However, samples do not suffer from distortion in this range of carbon content. The residual carbon in the incompletely debound samples reduces the optimum sintering temperature, with this temperature decreasing as the residual carbon content increases. The fully debound samples achieve a maximum densification at 1270℃ and the partially debound parts achieve quasi-full density at 1240℃. A carbon content of >2 wt% does not seem to affect the results, whereas carbon contents above 3 wt% lead to the formation of eutectic carbides at very low temperature, which results in a worsening of the mechanical properties, heterogeneous microstructures, and distortion of the components.
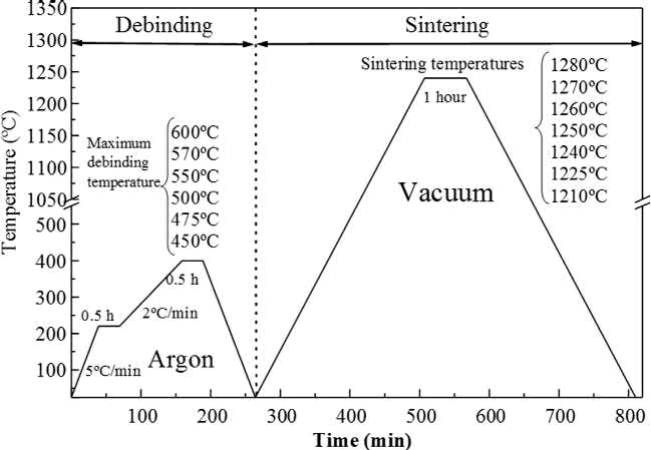
Fig. 14.12 Debinding and sintering cycles applied to study the effect of incomplete debinding processes in high-speed steel (HSS).
The microstructures of a correctly sintered HSS component and a component with an excess of residual carbon are compared in Fig. 14.13. The second effect is the widening of the sintering window by >20℃, as can be seen in Fig. 14.14, which is much wider than normally found for these kinds of steels. Careful control of the carbon content could help the industrial processing of these steels, although the relationship between the carbon content and the atmosphere should be studied in depth for each particular case. The size of the samples and the reproducibility of the process are the main factors to extract any industrial advantages from these facts.
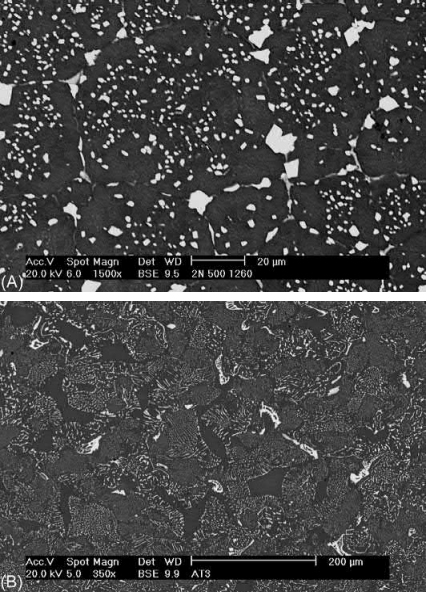
Fig. 14.13 Microstructure comparison between an M2 HSS component with 2 wt% of residual carbon with an optimal distribution of carbides and component (A) with >3% wt of residual carbon with the formation of undesirable eutectic carbides (B).
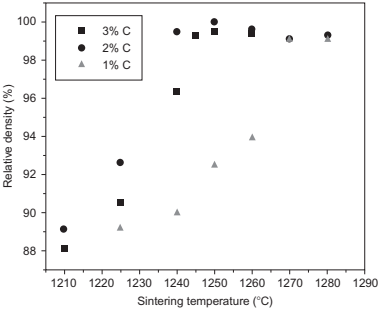
Fig. 14.14 Sintering curves for samples debound at different temperatures and then, different residual carbon contents.
In general, the use of H2-rich atmospheres leads to decarburization of the samples, therefore, the use of vacuum or H2-N2 mixtures is often more appropriate. Indeed, the use of a nitrogen-rich atmosphere is of interest from a technological and economic perspective as it permits the continuous production of HSSs as well as other important benefits, such as changes in the final microstructure. The presence of nitrogen during sintering results in the substitution of MC carbides by fine MX coarsening-resistant carbonitrides whilst leaving M6C carbides unaffected. This effect has rather greater consequences in the case of high-vanadium HSS grades. Thus, as described by several researchers, the great affinity of vanadium for nitrogen results in a sequence of reactions [MC + N!M(C,N) +C!MN +C] that leads to the precipitation of vanadium nitrides dispersed through the matrix, which is increased if the sintering takes place at a higher nitrogen pressure. This implies a further factor that must be considered when designing the sintering process for HSS components in order to be able to control the volume fraction in the liquid phase as, if these reactions take place, the amount of carbon available increases and therefore the sintering conditions change.
HSS has been used as a cutting material and frequently competes with cemented carbides in some applications. The addition of several types of reinforcement to HSS during PM generally results in a decrease in the sliding wear rate, thus allowing for a broader field of applications. These findings led to the production of HSS-based metal matrix composites (MMCs) by MIM. This production route offers many unique advantages for the mass production of small and complex parts. The main purpose of steel-based composites isto improve wear resistance; therefore, the addition of carbides and nitrides to feedstock compositions to meet this objective has notable consequences on the carbon control of the system, as described briefly in the following section.
The addition of carbides both improves the mechanical properties and helps to obtain a homogenous distribution of the liquid phase thanks to the addition of a carbon source being, in industry, more reproducible than the use of residual carbon. Recent results concerning the production of reinforced M2 HSS demonstrate that carbides have a powerful grain growth inhibiting effect and that these reinforcements decrease the optimum sintering temperature and broaden the sintering window. Furthermore, it has been demonstrated that the inhibiting capacity differs depending on the type of carbide and its reactivity with the steel matrix. Thus, in the case of the addition of vanadium carbide (VC) (Fig. 14.15), which has a great affinity for nitrogen, the reduction of the sintering temperature, the broadening of the sintering window, and the precipitation of vanadium nitrides are especially significant and are therefore of great interest for many tool applications.
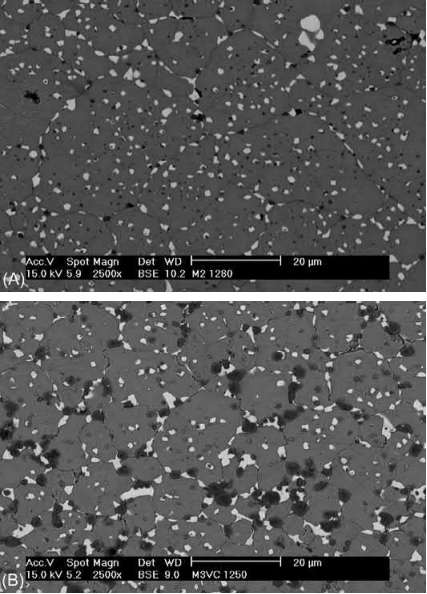
Fig. 14.15 Microstructure of M2 HSS sintered under N2-H2 atmosphere at 1280℃ (A) and M2 HSS reinforced with 3wt% of VC sintered at 1250℃ (B).
Besides the increase in wear resistance of the components, the most important aspect of the microstructural evolution seems to be the relationship between the carbon content and the nitrogen absorption. Some actual M2 HSS components produced after a strict control of carbon content during debinding and sintering are shown in Fig. 14.16.
Fig. 14.16 M2 HSS components for the textile industry with hardness >64 HRC.
Fig. 14.16—Cont’d
Stainless steels are the most common metal produced by MIM, followed closely by iron-nickel steels. Stainless steel parts in both the 300 (austenitic) and the 400 (ferritic or martensitic) series are produced from powders. The 300 series austenitic alloys are typically used in applications that require good corrosion resistance (303L, 304L, 316L), whereas ferritic grades are used in applications that require magnetic properties or good thermal conductivity and/or durability and in applications that involve thermal cycling (409, 410, 430). In the martensitic form, these steels are used in wearresistant applications although the martensitic grade has the lowest corrosion resistance of all P/M stainless steel grades (420, 440). Two special grades, viz., duplex stainless steel with a ferritic-austenitic microstructure, and phase-hardened (PH) stainless steel, which can be strengthened by solution treatment, are used very frequently. Duplex stainless steels have higher mechanical strength and improved resistance to stress corrosion cracking than austenitic grades. Their toughness is higher than that of the ferritic steels but slightly lower to that of the austenitic ones. The PH stainless steel (14-4PH, 17-4PH) presents high strength and good corrosion resistance. This combination of properties makes these steels very common in MIM and has widespread applications.
The corrosion resistance of sintered stainless steels, which is a key property for the majority of uses, depends on several factors, several of which are related to sintering. The optimal mechanical properties of MIM components require densities in the range between 97% and 98% of the theoretical value. These high densities are achieved by using extremely fine spherical powders and by sintering at high temperature. For stainless steels, these temperatures range between 1120℃ and 1350℃ for times of between 30 and 120min under a protective atmosphere or vacuum. During sintering, it is vital to avoid contamination and minimize the presence of precipitates of chromium carbide, chromium nitride, and silicon oxide in the microstructure, as well as to control the formation of surface oxides and/or nitrides on cooling. To avoid these detrimental processes, the carbon content should be very low as its presence lowers corrosion resistance. The main reason behind this phenomenon is the possibility of intergranular corrosion at the grain boundaries of stainless steels. The solubility of carbon and nitrogen into the matrix at room temperature is much lower than at high temperature. Carbon and nitrogen therefore precipitate as carbides and nitrides at the grain boundaries during cooling. In addition, the diffusion rates of carbon and precipitated carbides are higher than the diffusion rate of chromium in the matrix. The great affinity of carbon for chromium therefore leads to the formation of chromium carbides, thereby producing a decrease in chromium concentration in those areas adjacent to the carbides. These areas are susceptible to corrosion. This fact suggests that it is important to remove the lubricant in conventional PM to prevent diffusion of carbon into the part. The carbon content is even more critical in the MIM processing and is therefore controlled during the debinding process. Binders can add up to 5% carbon to the part after molding, whereas the maximum allowed carbon content in the final sintered part can be 0.03% or less for sintered stainless steel. There is a correlation between the degree of carbon control and the oxygen content during binder elimination as oxygen assists in carbon extraction via formation of CO and CO2. However, the grain surfaces can be oxidized by the binder and the resulting oxides reduced again at sinter temperatures by carbon diffusing to the surface oxide. This process therefore must be strictly controlled.
The main stainless steels used in commercial MIM applications are 316L and 17-4PH. A 316L is an austenitic steel known for its corrosion resistance and 17-4PH is a precipitation hardening grade with reasonable corrosion resistance and a much higher strength than the austenitic stainless steels. Another promising stainless steel, viz., nickel-free austenitic stainless steel, arose from the need to avoid the release of nickel into the organism during biomaterial applications. The effect of residual carbon on each grade will be described in the following section.
Even though the carbon content in the austenitic steel 316L must be kept very low in order to ensure maximum corrosion resistance, a very small quantity of carbon could be beneficial for reducing the oxide on the powder surfaces, frequently silicon oxide. At this point, the debinding atmosphere is very important to control the residual carbon. The most used atmospheres are hydrogen, nitrogen + hydrogen, argon, nitrogen, or combination of inert atmosphere with small percentages of air or oxygen to contribute to the burnout of the binder. The interaction between the atmosphere and the powder could produce unpredictable results. Although the presence of hydrogen should lead to the reduction of the residual carbon in normal conditions, if the powder presents oxides (very common in brown parts), the hydrogen reacts with the oxygen which is not available to react with the residual carbon. The carbon content in parts debound under inert atmosphere could be lower which is especially relevant for stainless steels.
In general, the best results are obtained upon presintering at 800℃ under a hydrogen atmosphere, followed by sintering at a maximum temperature of between 1300℃ and 1390℃ under hydrogen or vacuum. Industrial experience suggests that 316L with a carbon content above 0.06% contains large pores and is less corrosion resistant (Fig. 14.17).

Fig. 14.17 Microstructure appearance of 316L component after industrial processing when the carbon content is above 0.06wt%.
The residual carbon content has a strong influence on the microstructure of injection-molded 17-4PH. Indeed, it has been demonstrated that carbon from the debound parts remains even after sintering. A relationship between carbon content and volume fraction of austenite has also been reported.
It can be seen in Fig. 14.18 that the volume fraction of austenite increases with carbon content, and increases abruptly over 0.1 wt% of carbon. There is also a relationship between the amount of austenite and the final mechanical properties of the sintered and aged compacts, which present lower hardness at higher carbon contents, with an abrupt decrease above 0.1 wt% of carbon. Changes in the microstructures are detected when the residual carbon content is varied. As the carbon content decreases, the microstructures change from predominantly austenite and martensite to martensite and δ-ferrite. These results lead to analysis of the contribution of δ-ferrite in the observed variations of properties. These variations arise because of the δ-ferrite phase formed during sintering. Thus, a higher amount of δ-ferrite is detected as the residual carbon content decreases because of the transformation γ!γ+δ that occurs at lower temperatures. The presence of this δ phase also has an important effect on the densification process. Thus, the δ/γ interphase boundaries contribute to the mass transport and this phase also increases the overall atomic diffusivity, both of which contribute to an increase in the densification of the compacts as the residual carbon decreases as it shown in Fig. 14.19, where the maximum densities are achieved at the highest debinding temperature.
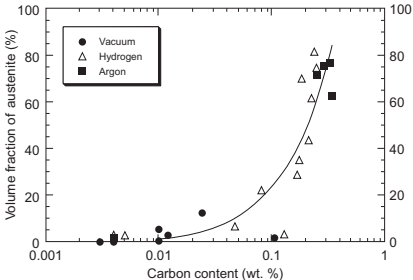
Fig. 14.18 Relationship between the volume fraction of austenite and the carbon content for sintered compacts in various atmospheres.
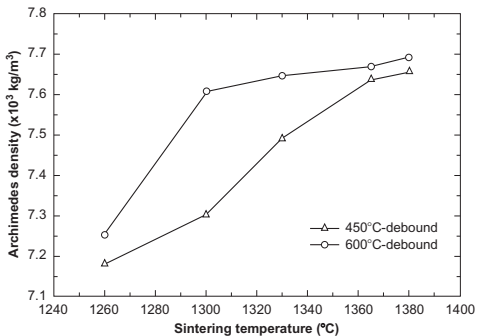
Fig. 14.19 Archimedes densities of PIM 17-4PH sintered at different temperatures debound at 450℃ and 600℃ to modify the residual carbon content.
A similar beneficial effect of δ-ferrite, because of residual carbon, has been reported in the sintering of duplex stainless steels. Furthermore, the mechanical properties of 17-4PH components with carbon contents above 0.08% have much lower mechanical properties and decrease the weldability. An example of optimized component for the medical industry made of 17-4PH with a carbon content under 0.08 wt% is shown in Fig. 14.20.
Fig. 14.20 Medical device made of 17-4PH stainless steel by MIM (14 g)
The production of high-carbon martensitic stainless steel, such as 440℃, by MIM has rarely been reported, mainly because of the rapid sintering densification that occurs during SLPS. Indeed, this process is often limited by the narrow processing window available to attain densification without distortion. Recent studies have shown that it is possible to obtain distortion-free components by MIM by accurately controlling the oxygen and carbon content. These steels have a higher amount of carbon that allows for conventional heattreatment by quench and temper. In this case, carbon has been found to decrease the liquid temperature and to enhance the sintering kinetics as more carbon means more liquid in the sintering phase. This liquid film could subsequently provide a viscous resistance to grain movement, thereby contributingto the rigidity ofthe structure during SLPS. A problem arises, however, when the distribution of the liquid is not uniform as the range of carbon contents throughout the sample lead to nonuniform shrinkage. The oxidation-reduction reactions between the carbon and oxygen present in the components during sintering decrease the carbon content and make the carbon distribution nonuniform. The reduction of oxygen content that occurs because of the deoxidation process after debinding in H2 allows components with better shape retention to be obtained. The content of carbon in this family of steels has an important effect on the shape retention.
Although the production of nickel-free austenitic stainless steels by MIM may appear unusual, the development of such materials is vital to obtain implants with the mechanical properties and price of stainless steel but with the ability to be used in long-term implantation. The elements that stabilize that austenitic microstructure and which could substitute nickelinclude cobalt, carbon, nitrogen, manganese, and copper. As this chapter is dedicated to the effects of carbon on steels, it is of interest to highlight the widely differing opinions available in the literature which indicate that this matter is only likely to be resolved with more experience in the use of this special stainless steel. One such view suggests that the carbon content should be kept to a minimum (as is the case for other stainless steels), whereas the contrasting point of view notes that the activity of carbon decreases significantly with nickel content, therefore, the austenitizing effect is more remarkable. The effect of residual carbon in such novel steels therefore remains to be clarified.
Low-alloy steels are generally used for structural applications. They are ideal for applications that require hardness and strength. These alloys include MIM-4140, MIM4340, MIM 4605, and MIM 52100 and those of FeNiC with different contents of Ni: Fe2NiC or Fe8NiC. These alloys are frequently heat treated to maximize their properties. These thermal treatments include different processes of quenching and tempering. The quench-and-temper heat treatments are processes that offer various strength and wear-resistance properties, then the resultant parts could be used in a wide range of sectors including automotive, consumer products, and firearms at modest cost.
The carbon content of these alloys could vary in the range from 0.35 wt% to 0.8 wt% (ISO 22068:2012, 2012). The powder contains these amount of carbon or slightly higher in order to keep the carbon content in the target specifications after the whole MIM process. To ensure a minimum loss of carbon content, the debinding and sintering atmosphere most commonly used is pure nitrogen. To minimize the prices of these products and optimize the properties, different initial powders have been tested to prepare the feedstock. Different experimental studies described that it is possible to use powders from different routes: prealloyed (PA), MAs, or using carbonyl iron powder (CIP) with the additions of the rest of alloying elements. Different studies have demonstrated that the use of PA and MA powders lead to improved mechanical properties, better control of distortion, better control of chemistry, and cost advantages (Coleman et al., 2012).
Another suitable possibility to increase the final properties of MIM parts of lowalloy steels consist of modifying the proportion of the elemental powders. For example, varying the nickel content (increasing up to 6 wt%) is possible to produce steel with superhigh strengthening properties achieving 2000MPa of tensile strength without loss of ductility. Moreover, it is possible to produce successfully steel with reduced amounts of Ni, substituting it by another less harmful element as Mn, keeping tensile strength of 1570MPa for the heat-treated samples. In all the cases, the sintering atmosphere of N2 or H2-N2 has to ensure a carbon content around 0.4 wt% in the final parts. The subsequent thermal treatments of reheating, quenching, and tempering are usually carried out in argon atmosphere to avoid oxidation and maintain the carbon content.
Cemented carbides are one of the most important groups of sintered tool materials due to their high hardness and outstanding wear resistance. This combination of properties is a result of their composite nature, whereby brittle refractory transition metal carbides (WC, TiC, TaC, Cr3C2, or Mo2C) are combined with a tough binder metal (generally Co, but sometimes Ni or Fe). Carbon control is the most important issue during manufacture of WC-Co cemented carbides by PIM as it determines both their mechanical properties and dimensional stability.
The carbon present in these alloys is critical as even small fluctuations in carbon content can alter the microstructure of cemented carbides producing changes in the properties as well as distortion. Thus, low carbon content gives rise to the formation of a brittle η phase, whereas excess dissociated carbon appears as graphite; both these new phases decrease the strength and hardness. To evaluate the microstructure dependency from the carbon diffusion and to define the role of the carbon content in the interfaces between carbides and cobalt, it is useful the measurement of the carbon compositional variations as a function of depth from the surface. Spectroscopic techniques are available for quantitative analysis of carbon, or for measuring compositional depth profiles as XPS, optical emission spectrometry (OES), and electron probe microanalysis (EPMA).
Studies in this field have tended to analyze the effect of the atmosphere used during the manufacturing process to control the carbon content. The debinding and sintering processes are especially long in the production of cemented carbides. Different atmospheres, such as a protective argon atmosphere during debinding, a presintering treatment in vacuum and sintering under argon, N2, H2, or a mixed atmosphere, could be combined in conventional processing methods (Upadhyaya, Sarathy, & Wagner, 2001). However, an N2 atmosphere would not remove the binder completely and an H2 atmosphere could result in decarburization if the debinding temperature exceeds 450℃, which in turn would result in inconsistent properties and dimensional control, as carbon control is even more difficult under such atmosphere. At temperatures above 450℃, the carbon in the brown parts could react with existing oxygen to form CO2 and further react with H2 to form CH4-producing decarburization of the samples:

Some studies have found that thermal debinding under a 75% N2/25% H2 atmosphere balances the decarburizing effect of H2 and the carburizing effect of N2, thereby resulting in an appropriate carbon control.
Another observed effect when the debinding is made under H2 atmosphere is the reactivity between the carbide and wet H2 atmosphere. In these conditions, the loss of carbon increases quite markedly (Spriggs, 1970). At temperatures between 00℃ and 450℃ the WC, for example, would react with H2O and form CO or CO2, producing the decarburization of the specimens:

The decarburization can be more serious if small amounts of W react with H2Oproducing WO2: As well, the loss of carbon could occur if there is any oxide in the mixtures because at temperatures around 600℃ it is possible to observe reduction of these species:

Although several steps can be used during thermal debinding to shorten the debinding process, it has been shown that the best carbon content control is achieved using a combination of solvent-based and thermal debinding (better than only thermal debinding) under 75% N2/25% H2 or vacuum debinding whilst maintaining the process at a lower temperature for a longer time. This method makes it easier to manufacture injected hardmetal parts with high transverse strength that require a stoichiometric quantity of carbon content in a narrow margin, usually close to 6% without carburization and decarburization.
One of the main current research topics in the field of cemented carbides concerns the development of new composites with partial or total substitution of the traditional cobalt binder by other cheaper and less toxic materials. A deeper knowledge of the phase diagrams of these new composites is therefore essential to obtain the desired final phase composition and to select the appropriate sintering cycle conditions, with carbon content being one of the main issues to be clarified. These advances will allow soon the development of new materials to be produced by MIM technique.
Contact: Cindy Wang
Phone: +86 19916725892
Tel: 0512-55128901
Email: [email protected]
Add: No.6 Huxiang Road, Kunshan development Zone, JiangsuShanghai Branch: No. 398 Guiyang Rd, Yangpu District, Shanghai, China