Nearly, all materials are sensitive to carbon. In fact, it is the most important issue during the metal injection molding (MIM) manufacture of some materials as even minor fluctuations in carbon content can alter the microstructure. Carbon contamination from the binder, which is a carbon-based chain that must be properly removed during the debinding step, is an inherent problem in the powder injection molding process. Its influence has a strong impact on the sintering process, microstructure, and mechanical properties of metals.
The influence of residual carbon may, however, vary significantly depending on the binder system. The sintering temperature and carbon content are the most important process variables in tool steels since they dictate the volume fraction of the liquid phase. In general, fractions of residual carbon help the densification process, thus allowing a better control of the process and an increase in the final hardness. In contrast, in cemented carbide systems, it is important to achieve an accurate control of the carbon to avoid carburization or the production of brittle phases. In other systems, such as most stainless steels, magnets (Fe-Si, Fe-Ni), or titanium alloys, the carbon content should be kept to a minimum as carbon contamination can lead to unacceptable mechanical properties and negative modification of the densification rate.
Carbon is an important aspect of the debinding process and depends strongly on the kind of binder system used. The large number of different binders therefore results in a complex approach to understanding the decomposition mechanism. A binder may be removed using any of a number of methods, and sometimes by a combination of several methods. The most commonly used process, which results in degradation of the polymer, is known as binder burnout and produces the so-called "brown" part. The binder contains a high level of carbon and therefore debinding requires an appropriate selection of the heating rate, temperature, time, and atmosphere to extract the binder and control the residues, the interactions during heating, and the formation of reaction products.
To effectively monitor and control carbon content, it is essential to understand the different sources of the carbon in the samples. The main source of carbon originates in the starting powder, other sources are the type of binder used and the specific system of decomposition of each particular binder component. This is key to understand the variety of reactions, which could be involved in each case depending mainly on the family of polymers that form part of the binder system and depending as well as of the rate and when the reactions take place. To better control this complex situation, there are different methods of monitoring the carbon content in the different steps of the process. The most direct tool is the measuring of carbon content in the brown and final parts using an elemental analyzer of carbon. However, this tool has to be used in conjunction with other analytical tools to understand the reactions and the reaction rates during the debinding and sintering steps and to be able to optimize the parameters to adjust the desirable content of carbon. One of the most common tools to determine the required debinding parameters is thermogravimetrical analysis (TGA). This tool allows the monitoring of sample weight with the increase of temperature. It is possible to program different heating rates or the use of different atmospheres. Analysis of the results allows the user to design the right debinding cycle controlling the residual weight of polymer, and thus, the carbon content. Theoretical models allow the decomposition behavior of polymers and the residual carbon left in the powder compacts to be predicted, thereby helping the development of optimized cycles. Other interesting tools used are the Fourier transform infrared spectrometry (FTIR) and mass spectrometry (MS) of the gas resulting of the debinding process, which allows the specification of the reactions that are simultaneously taking place, and monitoring in situ the equilibriums that occur during the process.
The above tools permit the measurement of the reactions that occur and the carbon content, which assists with the adjusts to the most convenient parameters after several trial-and-error processes in different experimental conditions. Nevertheless, the true control of the carbon content occurs due to the two key factors: the atmosphere and temperature selected. The selection of the atmosphere at different temperatures implies the formation of different species during the debinding process. A better understanding of these reactions would ensure a better choice of the binder system, better and more stable control of the carbon content, and, in the end, reduce the optimization steps and the trial-and-error process during the adjusting of the debinding parameters. In general, as the reducing character of the atmosphere increases, an increase in the final carbon content is observed. This fact is attributed to the reaction between the hydrogen and the oxygen on the powder preventing the reactions between the carbon and the oxygen that leads to a reduction of the carbon content. In other words, the burnout products change from carbon-oxygen predominance to hydrogen-oxygen predominance as the reducing character of the atmosphere increases. The debinding temperatures also change upon changing the atmosphere. A balance between minimum debinding time, compact integrity, and suitable carbon content must therefore be achieved during the industrial processing.
Finally, it is important to correlate the carbon content to the material's properties and its dimensional control. The different debinding methods applied have a key influence on the final dimensional control, due to its relation with the carbon residues and the stability of the dimensions of the parts during its processing. Also, several authors have found a strong influence of carbon content on microstructure development. An approach to different metallic system facilitates understanding of the combination of diagram-phase modifications with the carbon content; formations of new phases are crucial to obtain different mechanical and functional properties. Thus, the final behavior in service of the different alloys is closely related to an accurate carbon control during the processing of the component.
In most alloys, carbon control has been achieved by adjusting carbon potential in the sintering atmosphere. In MIM, the presence of a binder component that is a source of carbon, iron oxide that can decarburize, the complex atmosphere composition that produces different gas-solid reactions, the CO/CO2 and H2/CH4 ratios, and the different effects caused by the residual carbon in the different alloys make the carbon control a multifactor question. Carbon control is a complex issue of balance between: the initial carbon content of the initial powders, the reaction rate of decomposition and the reaction rate of the residual carbon with the debinding atmosphere and, finally, and no less important, the interaction of the powder and the residual carbon, in the different residual state that could appear, with the sintering atmosphere.
Throughout the debinding process, the polymeric binder decomposes into hydrocarbons ranging from C1 to C6. These molecules may themselves be reactants and break further into small hydrocarbon chains by the radical processes. The process consists of thermolytic bond cleavage, recombination reactions, and volatilization of lowmolecular-weight products.
The thermal degradation mechanisms of different types of polymers lead to the production of different amounts and kinds of carbonaceous residues. The use of FTIR and MS of the gas resulting of the debinding process could permit a complementary understanding of the processes although their use is not yet so extended and the interpretation of the results is not so easy. The current situation is that without knowing the amounts of each species and the existing gases, it is very difficult to predict which reactions are occurring but it is possible to give some general ideas. For example, the products of a depolymerization-type polymer such as polyacetal (POM) or polybutyl methacrylate (PBMA) are completely different than the residues produced by randomtype polymer or polyolefin such as polypropylene (PP) or ethylene-vinyl acetate (EVA) as it is described by Kankawa.
When POM or PBMA decomposes, they do not produce any carbon residue in either air or nitrogen. They decompose into monomers of the main chain which are easily evaporated at a low temperature (300℃), as illustrated in Fig. 14.1A. Monomers are sequentially lost at the chains end. However, polyolefins or random-type polymers decompose to low-molecular-weight compounds at high temperature and require longer time. The degradation can be described by a random scission of the chain. It could produce some carbon double bonds that require even higher decomposition temperatures (600℃) (Fig. 14.1B). In spite of these results, the use of depolymerization-type polymers as a single component of the binder could lead to the production of samples with cracks and holes during thermal debinding in air because of the faster decomposition rate. A combination of both kinds of polymers seems to lead to better specimens. In this case, the best results are obtained using a sequence of catalytic debinding to eliminate the polyacetal under NH3 atmosphere and leave the polyolefin, followed by a thermal debinding cycle under a controlled atmosphere. A more detailed description of the binder decomposition mechanism will be gone in detail later in this chapter in order to understand the different types of residue that acts as carbon source in the MIM process.
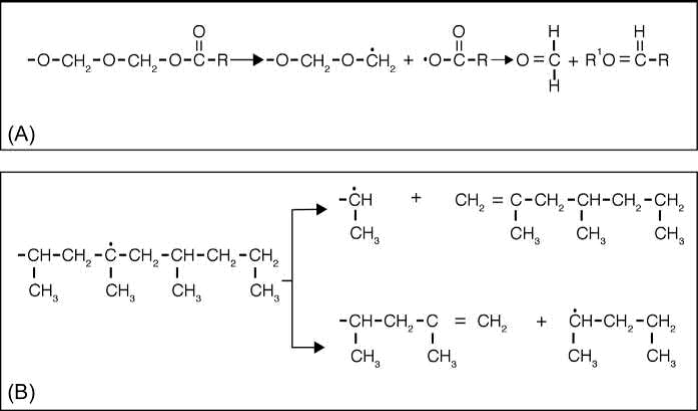
Fig. 14.1 Degradation mechanism of polymers: (A) depolymerization type (polyacetal) and (B) random decomposition type (polypropylene).
It is important to be aware of the main tools available to control the carbon content throughout the process from the green part to the final sintered part. The main tool used during carbon control is the elemental analyzer designed for wide-range measurement of carbon content of metals, ceramics, and other inorganic materials using the combustion and infrared detection technique as those from LECO, BRUKER, HORIBA, etc. This analyzer allows for carbon control after the debinding and sintering in most of the alloys. In some cases, it is necessary to modify the calibration range of this method by modifying the amount of the sample and using special carbon test standard samples, but it is adaptable to most alloys.
Another way to design debinding cycles for carbon content control is the use of TGA as it was described above by monitoring the sample weight as the temperature increases. The weight loss as a function of time shows an almost linear decrease up to a certain value, after which the weight loss becomes slower. This deceleration of the weight loss during binder removal for nearly equiaxed shapes is mainly due to geometrical effects. The explanation could be that the binder interface advances at almost constant speed in the molded sample, the reaction surface decreases and hence the rate of weight loss decreases.
TGA is suitable for studying the decomposition behavior of the polymers used as binders. Indeed, by choosing different atmospheres and a constant heating rate, it is possible to distinguish the effect of the atmosphere on the decomposition rate of each binder. As an example, Fig. 14.2 shows the TGA of a binder of two components, paraffin wax (PW) and high-density polyethylene (HDPE). The TGA was programmed using a constant rate of 10℃/min under N2 atmosphere. It can be observed that the PW starts to decompose near to 220℃ and at 450℃ the main weight losses takes place corresponding with the degradation of the HDPE. Based on these results, it is possible to design a debinding cycle for the feedstock.
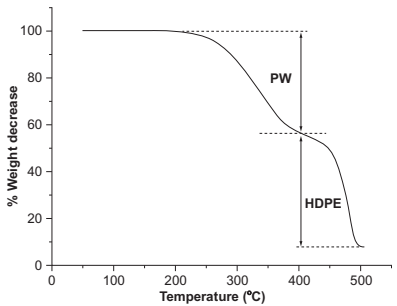
Fig. 14.2 Thermogravimetrical analysis (TGA) of a two components binder.
The debinding schedule comprises different steps with two main elements: heating rate ramps and isothermal stages. A combination of these elements will produce defectfree parts. In the first step of the heating cycle, a high rate of 5℃/min can be chosen before any weight loss occurs. At 220℃ where the paraffin starts to evaporate, an isothermal step has to be selected to favor a slow elimination of this component. Between 220℃ and 500℃, the main weight loss occurs progressively and the heating rate is reduced by 2℃/min. These heating rates were optimized because high heating rates produce the presence of external blisters and cracks, and slow heating rates extend the process. The last isothermal step is considered as the "maximum debinding temperature" and it determines if there is some residual binder left over. The optimal thermal debinding cycle has to be applied directly on the feedstock to check the viability becausethe presence of metal powder could slightly modifythetransitiontemperatures. Fig. 14.3 shows the correlation between the debinding cycle and the TGA applied directly over the feedstock, showing what a maximum debinding temperature left, which in this case is 1 wt% of residual carbon in the sample.
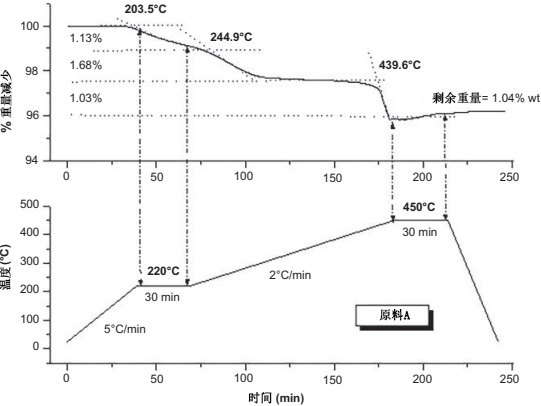
Fig. 14.3 Correlation between the debinding cycle and the thermogravimetrical analysis for a feedstock based on PW and HDPE
Further information regarding which reactions are taking place and which gaseous species are involved in the process can be obtained by analyzing the resultant gas using FTIR and MS or the combination of TGA and FTIR studies. This approach allows for the simultaneous evaluation of bond breaking and product formation during decomposition of the polymeric binder, as, during the decomposition process, the gases are collected and analyzed. These systems make it feasible to study reaction pathways for the formation of gaseous products and the degradation mechanisms of materials during pyrolysis. In addition, it provides the residual weight of each kind of binder, essentially the residual carbon, and an in-depth understanding of the decomposition mechanism can be obtained. Using an appropriate software a kinetic model, in which time-temperature-gas relationships are shown, can be obtained from these studies.
Among the debinding variables related to the carbon control, the debinding route, thermal cycle, debinding atmosphere, and gas flow rate are key issues. Indeed, use of a combination of different debinding processes generally leads to a better control capability than a single-stage process such as thermal debinding. The large number of different binders and different debinding methods (solvent, thermal, wick, catalytic, vacuum, etc.) results in a complex approach to the decomposition mechanisms. The most commonly used combination to ensure appropriate carbon control involves a solvent debinding step, which results in partial removal of the binder by the solvent, followed by a thermal cycle to burn out the residual binder under a protective atmosphere. This step usually is a combination of evaporation, liquid and gas migration, pyrolysis of polymer, and heat transfer in porous media.
The solvent extraction consists of diffusion of solvent (usually organic) into the binder, dissolution of the binder, interdiffusion of the solvent and the binder, and the outward diffusion of the solution to the surface. The use of solvent debinding as complementary step of the debinding in industrial processing has resulted in better dimensional and carbon control. In recent years, the use of water debinding has been an attractive method due to concerns over environmental pollution, cost effectiveness, and manageability. The degradation in water has the same basis as the dissolution of the binder in any other solvent although the effectiveness in the elimination of residual binder has only been studied in-depth for a few systems. One of the most common polymers uses as backbone in water-soluble systems is the polyethylene glycol (PEG) and its degradation process has been recently studied by different authors in MIM products. These studies were performed via FTIR and other conventional methods such as TGA and differential scanning calorimetry (DSC). They reveal possible chemical interactions between the polymer constituents and the powder constituent of the feedstock. All the studies corroborate the formation of a complex mixture of decomposed products during the solvent and pyrolysis of PEG, which were previously observed in the FTIR bands. Moreover, they showed that the interaction between the PEG-based binder and powder depends on the nature of the powder, concluding that each case has to be analyzed. In some ceramic feedstock, active interactions were detected showing remarkable influence in the degradation behavior of the binder (retarding the decomposition). Furthermore, as a result of these interactions, it was possible to suggest an approximation of the optimal powder loading of the feedstock. In the other study, particularly in the case of feedstock based on Inconel 718 and PP-PEG binder, the use of PEG seems not only to decrease the viscosity but also to increase the ecology of the binder even allowing a clean degradation process with no detectable interaction between the metal powder and the binder. An important question to address is the evaluation of the amount of oxygen left by PEG systems in comparison with other polymers, due to possible increases in the decarburization, in the first steps of the sintering process, because of the residual oxygen and carbon interactions.
In the case of thermal debinding, the binder can be removed using different mechanisms or a combination of several:
‒ evaporation (observed in the case of low-molecular-weight binders);
‒ thermal degradation (which takes place in the depolymerization of the polyolefins that transform into low-molecular-weight substances that diffuse to the surface and finally evaporate); and
‒ oxidative degradation (in which the binder reacts with oxygen and is eliminated).
Debinding is affected by the nature of the individual binder components, the interaction between the different components and the interaction between the binder and the powder particles. When taking into account all these aspects in order to understand the source of the residual carbon after the debinding process, it becomes clear that binder removal by the first mechanism, viz., evaporation, does not leave carbon residues.
In contrast, thermal degradation occurs all over the compacts and the amount of residue depends on the molecular weight of the binder components. It is important to note, however, that if the components of the binder, or the binder and the powder, interact physically and/or chemically, the amount of residual carbon content can change completely. The binder residues left from debinding could be critical for structural, physical, or magnetic properties because modifying the carbon content of the system before the sintering step produces changes in the final microstructure, precipitation of new species, etc. The most of the desirable binder systems leave small amounts of residual components, mainly low-weight species or carbon. These species are usually very fine and they appear to be homogeneously distributed through the matrix, although their effect could change depending on the quantity of the residue and the kind of alloy. Most of these binder systems are designed to burnout completely during heating to the sintering temperature. Consequently, some stronger backbone polymers such as polystyrene, starch, or cellulose should be avoided as they tend to form graphite-producing carbon contamination which is difficult to repair even in the sintering step. Furthermore, due to incomplete debinding, both carburization and decarburization processes could be subsequently affected to the sintering step.
In general, the combination of solvent and thermal debinding results in lower carbon contents as compared to only thermal debinding. This is due to the dissolution of binder components during solvent debinding, which leaves an open pore structure for subsequent binder burnout . Moreover, after studying the sintering process for each system, and evaluating the carburization and decarburization tendency, a correct adjustment of solvent debinding (the same occurs with the catalytic processes) enables a good fitting of the final carbon content.
Oxidative mechanisms are used less frequently as most metal powders undergo severe oxidation at the debinding temperatures. Some companies apply debinding in air, up to the temperature at which the TGA curve shows weight gain of the powder as long as the TGA curve shows weight loss of the binder. This process helps to reduce the long debinding time cycles because oxidative degradation lowers the decomposition temperature of the polymers and accelerates their removal. The use of oxidative atmosphere could lead to an excess of carbon as well. This effect is observed because in air the excessive oxide formation on the powder surface may close the channels for the decomposition gases, producing incomplete thermal debinding. In these cases, debinding in air is restricted to a maximum temperature. Therefore, when the atmosphere used is nonoxidative, binder removal is mostly restricted to evaporation and thermal degradation processes.
One of the various models proposed to predict the residual carbon content after thermal debinding suggests that the residual carbon content is determined by the isothermal holding time at the "maximum debinding temperature" Lowmolecular-weight materials (such as wax) are easily removed by evaporation and therefore leave only minimal amounts of residual carbon. Polymer can degrade in a number of ways, including:
‒ chain depolymerization;
‒ random scission; and
‒ side-group elimination.
Chain depolymerization produces volatile molecular fragments that are practically monomeric. Polymers that decompose into volatile alkanes and alkenes (such as PMMA) appear to be some of the cleanest binders.
Random scission (as found for HDPE, PEG, or EVA) produces a wide spectrum of molecular fragments that contain little monomer therefore they frequently result in residual carbon formation. This is often due to cross-linking reactions between initially degraded chains. This kind of reaction and cyclization has to be considered as they both lead to the formation of nonvolatile carbonaceous residues. The residual carbon resulting from these processes generally has one of the two forms: pseudographitic carbon, which is composed of multiple polycyclic groups (similar to graphite), or highly branched carbon, which is nonaromatic carbon residue (see Fig. 14.4). The residual carbon content increases linearly with an increase in the logarithm of the molecular weight. Although this type of residue is easily eliminated in an oxidizing atmosphere, the limits on the use of oxygen in the presence of metallic powders, lead to a need to choose a binder that degrades into volatile components, has a complex composition that allows its progressive elimination (e.g., PP/LDPE/PW, HDPE/ PW/SA, PEG/PMMA, HDPE/PP/PW/EVA/SA, CAB/PEG, and EVA/PW/HDPE/SA) or includes additives that help to volatilize the polymer components in inert atmospheres to ensure low carbon residue contents in those materials where the carbon residue must be kept to a minimum.
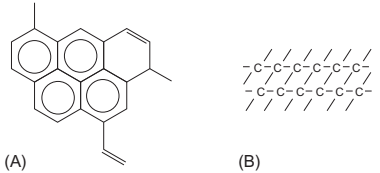
Fig. 14.4 Schematic illustration of the chemical structure of carbonaceous residue: (A) pseudographitic and (B) cross-linked
The final mechanism, viz., side-group elimination (e.g., found in PVB or PVA), also produces appreciable amounts of nonvolatile carbonaceous residues. Besides water or butanal, such polymers typically produce long chains containing some unsaturation and conjugation, which can subsequently undergo cyclization and cross-linking reactions that result in significant amounts of residual carbon.
A suitable binder must therefore be chosen on the basis of the requirements of the metallic system to be produced. For example, noncarbon containing materials such as copper-based alloys can be processed in hydrogen gas and eliminate any of this residual carbon as long as the metal does not react with the hydrogen to form hydrides.
Coupling agents or surface active agents are widely used to improve the interaction between the metallic or ceramic powder and the polymer components. Some additives, such as carnauba wax, act as lubricants at the die-walls decreasing the total viscosity of the feedstock and permit to increase the powder loading of the feedstock. Other additives, such as stearic acid, have been described as dispersant, reducing the viscosity of the mixtures when it is added directly to the feedstock. However, due to the chemical nature of the stearic acid, it has a polar end and nonpolar end, this additive is able to create chemical bonds between the binder and the powder surfaceswhich frequently leads to an increase in residual carbon in the form of other coupling agents such as aluminate, silane, and titanate, frequently used for improving the rheology of the mixtures (mostly in ceramic systems). To create the chemical bonds between the binder and the powder surfaces, it is necessary that stearic acid (C17H35COOH) is adsorbed onto the metal powder in a previous treatment in a concentrated solution. The metal powder is able to interact with the polar end of the stearic acid molecule and the binder interacts with the nonpolar part following the reaction in Fig. 14.5.
The formation of the monolayer of stearic acid, which covers the powder, can be detected using X-ray photoelectron spectroscopy (XPS) analysis because the carbon peak intensity increases compared with the powder without any coverage. This monolayer can be identified in FTIR analysis, as well, because appear a new peak characteristic of COO-new bonding and peaks of the appearance of water molecules. To determine the optimum amount of stearic acid adsorbed onto the metal powder surface, measure the stearic acid melting enthalpy of the observed peaks in the DSC curves. It can be observed that melting enthalpy increases with the SA concentration of the solution, achieving the saturation at a particular concentration of SA and the optimum percentage of stearic acid. Another possibility is to estimate the amount of surfactant that covers the specific area of the metal powder, forming a complete monolayer surface coverage, taking into account the density, the powder size and morphology, and the proportion of surfactant in the binder.
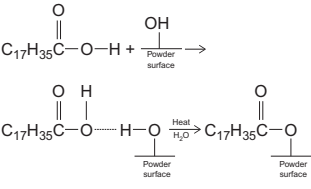
Fig. 14.5 Chemical reaction on surface of metal powder with SA.
Although the use of a surfactant improves the fluidity of the mixtures, the bonding with the metallic powder can alter the mechanism or the rate of binder elimination. TGA studies must therefore be performed in the presence of this powder as the shape and temperatures of the curve could suffer important modifications. Indeed, in some cases, burnout may be retarded as the small size of the particles hinders binder evaporation, or the presence of a powder can enhance the thermal degradation. This catalytic effect may be due to the described reactions between the metal powder surface and polymer or additives. For example, the activation energy for decomposition of EVA decreased in the presence of spherical powder 316L. Besides, certain oxide surfaces affect the decomposition behavior of polymers. In this sense, it has been reported that purer powders and larger surface areas (smaller particle size) increase the catalytic activity, thereby reducing the debinding time.
The cost of removing the binder is very high because it is usually a long process, and several aspects of the polymer removal process and predictions of the residual carbon left behind by different binder systems remain to be investigated in order to optimize this step of the MIM process. It is important to consider some particular factors that have to be analyzed in any case, such as the size of the part that dictates the ability of the atmosphere to interact with the binder. This fact means that thermogravimetric data from one specific system cannot be directly applied to another.
For these reasons, great efforts have been made to simulate these processes in order to anticipate the behavior of binder systems in the presence of metallic or ceramic powders and the taking into account of the particular geometry or the size of the part. It has also been reported, and some examples have been shown above in this chapter, that the interaction between the binder component and the powder surface leads to increased residual carbon. One way to quantify the activity of the powder surface is the so-called isoelectric point (IEP) and some experimental methods have been proposed in the literature to measure it in metal surfaces. In several investigations, the carbon content was smallest for powder with IEP close to 7 (neutral). The more acidic or basic a powder's surface, the more interaction between the powder and the binder and the higher the amount of residual carbon.
The true control of the carbon content comes from the combination of an appropriate atmosphere and temperature during both the debinding and sintering processes.
Regarding the debinding step, depending on the different atmospheres used during this process leads to the formation of different species changing generally from carbon-oxygen dominance to hydrogen-oxygen and hydrogen-carbon dominance as the reducing character of the atmosphere increases. The best results are obtained when equilibrium between the gas species is achieved by a combination of carburizer and decarburizer gases. However, although the chosen atmosphere may have a good equilibrium between CO/CO2 or CH4/H2, the production of gases on decomposition of the binder produce that the ratio of the gas mixture vary continuously, thereby making it difficult to ensure a constant composition. In general, the use of pure H2 leads to a minimal carbon content and lower decomposition temperatures, although this is sometimes counterproductive as it may result in severe decarburization of the resulting parts and hydride formation which is also undesirable.
If the debinding experiments are carried out in H2 atmosphere, the degradation can be attributed to the reaction of H2 with the binder (Eq. 14.1) but it is very common to observe decarburization processes following the same mechanism (Eq. 14.2):
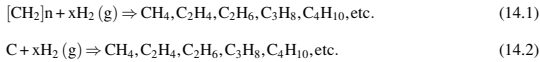
If the debinding take place in N2, Ar, or a vacuum, it is possible to observe other kinds of decarburization processes attributed to other reactions of the residual carbon with residual oxygen or some oxides present on the metallic powders:

If the debinding takes place in mixtures of H2-N2, the decarburization rate comes from a combined effect of both processes with a remarkable influence of the isothermal holding time, as a result of these processes being controlled by the diffusion of carbon in the metal phase.
In alloys where the initial carbon content is minimum or near to zero, the most successful results are obtained by an optimum hydrogen/nitrogen mixture atmosphere to ensure residue-free debinding and reduce the oxides but to avoid the severe decarburization effect of H2.
Another relevant industrial parameter that must be controlled during the debinding process is the temperature. Debinding temperatures change as a function of the atmosphere, thus meaning that a balance among the minimum debinding time, compact integrity, and suitable carbon content has to be achieved during industrial processing. Thus, while it may be more interesting industrially to use high temperatures to reduce the debinding time as the elimination rate increases considerably, an appropriate balance must be found. Indeed, higher temperatures can easily produce internal stresses due to vaporization of the binder occurring too quickly, which could cause the component to blister or crack. Moreover, higher temperatures could lead to distortion of the components due to viscous flow under gravity as the binder softens. It is therefore important to choose heating rates according to the decomposition stages of the different components of the binder to prevent the onset of defects.
In short, the debinding cycle, the atmosphere, temperature, and isothermal holding time at the maximum debinding temperature, must be tailored to the binder, and in industrial practice, the optimal hydrogen/nitrogen proportions must be individually determined. The models suggest that an adequate control of these parameters permit the prediction of the residual carbon content after debinding.
Industrial processes have to balance a minimum debinding time and compact integrity with a correct selection of the atmosphere according to the metallic system produced. The combination of different debinding processes, such as solvent and thermal debinding, usually reduces distortion of the components whilst allowing the duration of the debinding stage to be minimized.
Regarding the sintering cycle: this is a critical process in which severe carburization or decarburization can take place depending on several factors, mainly the atmosphere used during the process, but also the residual carbon left after the debinding, the nature of the powders and its partial oxidation after the debinding, which could lead to parts with completely different properties. The control of the final carbon content could be covered by the adjusting of the solvent debinding step, modifying the carbon content of the initial powders to compensate for an avoidable decarburization, or introducing modifications in the gases to change the reactions that take place.
Concerning the different atmospheres used in the sintering process of MIM parts, the most successful are: hydrogen, nitrogen, and nitrogen/hydrogen mixtures, even with additions of methane, which enable carburization and reduction at the same time, and vacuum. Argon atmosphere is not so frequently used because it retards the densification by filling the pores impeding a full densification of the components. Pure hydrogen is the preferred sintering atmosphere for stainless steels. The hydrogen suppresses the chromium evaporation which improves the corrosion resistance. Nitrogen or a combination of nitrogen and small amounts of hydrogen is a very adaptable atmosphere, used for most of the alloys. Vacuum, at least initially, is a neutral atmosphere which does not react with the powders, but in a sintering chamber with a diffusion pumped vacuum, low oxygen partial pressure could act as a reduction atmosphere for iron at typical sintering temperatures (Fig. 14.6). In whichever case, it is a successful atmosphere especially for tool steels and other sensitive alloys.
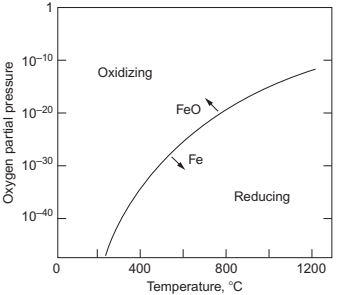
Fig. 14.6 The separation between iron and iron oxide is plotted as the oxygen partial pressure vs temperature, with oxidizing conditions above the line and reducing conditions below the line. Vacuum sintering at temperatures over 1200℃ typically occurs under reducing conditions. Reproduced with permission from PIM International.
Despite all of this, a determined atmosphere is not always the best option for a specific material. It is important to choose the atmosphere as a function of the final application of the products. For example, for some components, it is preferable to have a high carbon content which is homogenously distributed into the microstructure to increase the total mechanical properties. However, in other cases, it would be interesting to have components of the same alloy with a minimum amount of carbon to ensure high toughness, although a carburizing treatment could be applied later to increase the surface strength. An example of the resultant microstructure in both cases is shown in Fig. 14.7 that corresponds to Fe-8%Ni alloy sintered in N2 (Fig. 14.7A) and H2 (Fig. 14.7B) to obtain real components with those described characteristics.
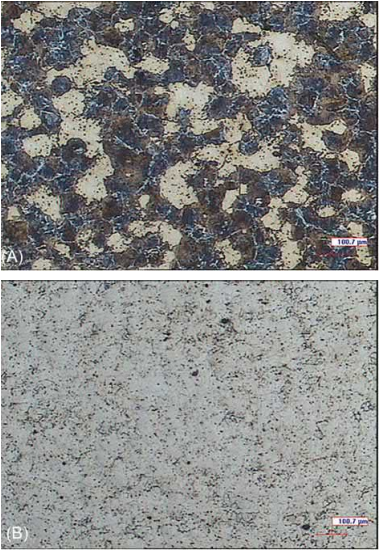
Fig. 14.7 Microstructure of Fe-8%Ni with (A) 0.47%C sintered in N2 and (B) 0.005%C sintered in H2.
The first reaction that occurs in the initial minutes of sintering is the reaction between oxygen and carbon to form carbon monoxide (CO) (Eq. 14.6). The reaction rate at a given temperature depends on the concentrations of oxygen and carbon in the solid material, diffusivities of oxygen and carbon, the equilibrium pressure of the reaction product, and the ambient pressure. Should the equilibrium vapor pressure of CO over the powders decreases because the carbon content and oxygen content are low, and should the impurities be reduced to low concentrations, the rate of CO evaporation would be expected to decrease.

Based on this preliminary knowledge of the oxygen and carbon interactions, nowadays, the experiments have determined that there is a correlation between the oxygen content in the powder or in the brown part (Od) and the grade of decarburization that take place.
The percentage of carbon lost (Cd) in weight is practically equivalent to the oxygen percentage of the initial sample (Od).

This fact led sometimes to the necessity of adjusting the carbon by adding graphite if the starting powders has small amounts of carbon and a certain amount of loss is unavoidable (Jinushi et al., 2002). As a result of this reaction, when the amount of carbon is not sufficient the part could appear excessively decarburized. This fact could lead to components which suffer critical deformations during the sintering process, as shown in the examples in Fig. 14.8.

Fig. 14.8 Decarburized real components that suffer critical deformations during the sintering process.
There are other cases which critical differences could be observed in the optimal sintering temperature if the initial powders of the same alloy have been obtained through different alloying processes. For example, Kearns observed in high-speed steel (HSS) alloys that the carbon loss during the sintering process, which determined the optimal sintering conditions, is predictable considering the origin [pre-alloy or master alloy (MA)] of the powder used, because the loss of carbon detected during sintering was related with the amount of oxygen present in the starting powders.
Depending on the atmosphere used, the thermochemical reactions involved are different going from oxidative to reductive reactions and from decarburization to carburization processes. In the common case of steels, one of the most critical reversible reactions is.

This reversibility means that a high concentration of oxygen leads to metal oxide and alternatively, at high temperatures a high concentration of metal oxide results in the formation of oxygen and pure metal.
This reaction could occur in vacuum sintering although the oxygen removal by pure dissociation from most metals is, in general, difficult. Degassing of oxygen is only possible if the sintering temperature of the material can be very high without the evaporation of the metal. At a typical sintering temperature, reduction requires an atmosphere with <10-13 parts of oxygen to avoid oxidation as could be extracted from Fig. 14.6. In fact, in vacuum sintering, the use of chemical reaction that involved the use of carbon deoxidation (Eq. 14.6) is the most effective method of reducing oxide in vacuum. This carbon deoxidation reaction is initiated at elevated temperatures and high vacuum levels.
Alternatively, the use of highly reductive atmosphere leads to reactions where practically any metal oxide (most frequently iron oxide) is reduced. In this case, the reaction progression is determined by the hydrogen/water vapor equilibrium, that is, the dew point, temperature at which water vapor will condense. This point gives information about the moisture content in the sintering atmosphere. So, to avoid the inversion of this reaction, a constant elimination of the vapor products from the furnace is vital.

Another important reducing specie involved is CO to produce CO2. Subsequently, an atmosphere will be able to reduce oxides if the CO partial pressure exceeds the CO2 partial pressure.

Finally, carburizing and decarburizing reactions are responsible for the final carbon content of the sintered part. The equilibrium could be studied by the map of carburizing and decarburizing (Fig. 14.9), where these opposite situations are given by the oxygen fraction and the carbon fraction.
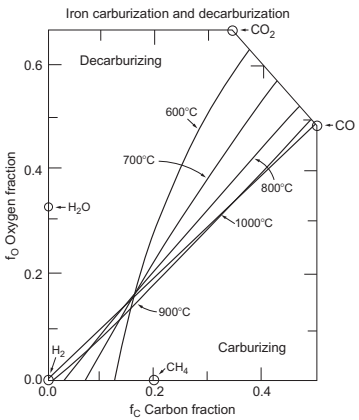
Fig. 14.9 Map of the carburization and decarburization regions for several temperatures and atmosphere compositions in terms of the oxygen fraction and carbon fraction
In this diagram, the main role is played by carbon monoxide, carbon dioxide, and methane. In general terms, the species that tend to produce decarburization are water and carbon while methane and carbon monoxide always cause carburization. Be aware that the amount of these gaseous and solid species is interconnected, thus, the total reactions that are taking place are very complex. Some species in equilibrium are constantly fluctuating during the sintering process due to the changes in temperature and to the appearance of new and/or reactive or products. The possible situations could go in different directions. Some possible examples could be: if the amount of carbon dioxide is high it will drive decarburization-producing carbon monoxide; if appear additional carbon coming from heaters elements of the furnace (in the case of graphite heaters) appears it will react with residual oxygen-producing CO that acts as reducing species; if any carbon source, graphite additions, or carbon coming from heating elements or residual binder is added intentionally, it will provide a useful carburizing environment; and if the water pressure is increased, it will produce an important decarburizing effect following equation.
As result of the balance of these reactions together the temperature and the control of water vapor, it is possible to go from totally distorted components to components with the exact required amount of carbon. In Fig. 14.10, components of 42CrMo4 steel appear decarburized (<0.2% carbon) and distorted on the left and with the correct amount of carbon (~0.38% carbon) correctly densified and keeping the correct dimensions.
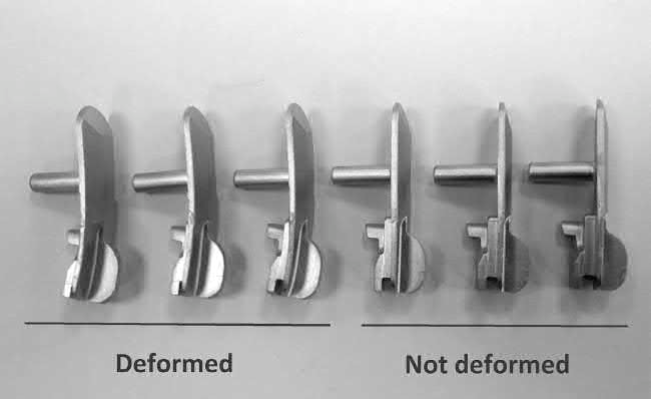
Fig. 14.10 Real components of 42CrMo4 steel decarburized (<0.2% carbon) and distorted (on the left) and with the correct amount of carbon (~0.38% carbon) correctly densified and keeping the correct dimensions (on the right).
Go beyond the understanding of these reactions, new control systems are playing an important role to tailor the atmosphere composition and optimize the sintering process. These control systems are based on sensors inside the furnaces coupled to a measurement equipment that monitor data of in terms of carbon content, temperature, oxygen content, hydrogen content, and other characteristics that overall are going to determine the reducing, oxidizing, carburing, or decarburing character of the atmosphere. Furthermore, these new control systems can program changes in real time to keep the final parts under controlled conditions, ensuring certain level of quality allowing to enter MIM technology in very sensitive sectors.
Contact: Cindy Wang
Phone: +86 19916725892
Tel: 0512-55128901
Email: [email protected]
Add: No.6 Huxiang Road, Kunshan development Zone, JiangsuShanghai Branch: No. 398 Guiyang Rd, Yangpu District, Shanghai, China