Although there are several different types of magnets, PM in general, and MIM, are effective for applications in which complex magnetic parts would otherwise require considerable machining. Furthermore, as densities approaching the theoretical density are possible when the components are processed by MIM, magnetic inductions close to the saturation magnetic induction are also possible. This means that magnetic inductions equivalent to those of wrought alloys are possible for alloys with a similar composition. In addition, it is possible to develop alloys by adding new alloying elements that cannot be considered using wrought fabrication technology.
Magnetically soft materials that are produced in large quantities include highpurity iron, low carbon steels, silicon steels, iron-nickel alloys, iron-cobalt alloys, and soft-magnetic ferrites. The magnetism of these materials is exhibited only while an external magnetic field is applied. Thus, in general, when a piece of iron is placed near a permanent magnet or in the magnetic field generated by an electrical current, the magnetization induced in the iron by the applied field is described by a magnetization curve obtained by plotting either the intensity of magnetization or the magnetic induction, B, as a function of the applied field, H. The behavior of any magnetic material can be defined by its hysteresis loop and B/H ratio, which is termed the permeability. This value indicates the relative increase in flux or magnetic induction caused by the presence of a magnetic material.
Three elements, viz., the ferromagnetic materials, iron, nickel, and cobalt, and their respective alloys, are truly magnetic. Indeed, the high magnetic saturations of iron and cobalt, together with their availability and price, mean that the commercial soft magnetic alloys used in the PM and MIM processing are commonly produced from highpurity iron or various ferrous alloy types such as Fe-2Ni, Fe-3Si, Fe-0.45P, Fe-0.6P, and 50Ni-50Fe.
Gas-atomized powders are normally selected to fabricate these parts because they are purer and finer, although iron and nickel powders produced by the carbonyl process are also widely used. Permeability, coercive field, and hysteresis loss are strongly affected by impurities within the alloy, with the impurities that are most harmful to these alloys including carbon, nitrogen, oxygen, and sulfur. For this reason, careful elimination of the binder is required to ensure minimal carbon content.
Most companies that produce magnetic parts use dedicated furnaces for sintering magnetic parts and select protective atmospheres such as N2-H2, vacuum, or argon, that do not include carbon to avoid contamination as even a small excess of carbon content (around 0.03 wt% C) seriously degrades the magnetic properties.
It is important to note that the formation of structural defects is common when significant densification occurs prior to complete binder burnout. The binder used in this system should decompose and the channels should be opened below 500℃ as densification starts above this temperature and the carbon content after sintering remains close to the residual carbon after debinding (Fig. 14.21). The process of closing the pore channels reduces the reaction area during densification, thereby reducing the decarburization rate.
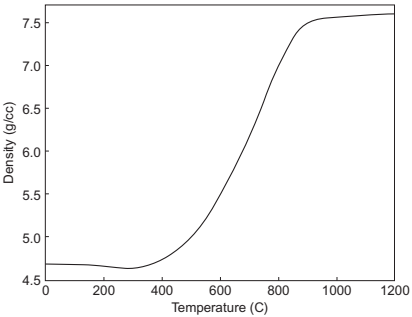
Fig. 14.21 Densification of Fe-2Ni as a function of sintered temperature in hydrogen.
Taking into account that the initial particles do not present significant oxidation, atmospheres containing high hydrogen concentrations (around 40%), which could help to remove the excess carbon, can be used to ensure a carbon content similar to the initial value. This atmosphere allows maximum values of hardness and strength to be achieved with a microstructure close to that of pure pearlite.
The decarburization process is influenced by other parameters such as the gas flow rate and the holding time, which are critical not only because the carbon content changes but also because the carbon distribution through the sample may not be uniform when the gas flow rate is too low, for example. The use of vacuum conditions is also common, in which case commercial carbonyl iron grade powder coated with SiO2, such as the BASF-OS grade CIP, is frequently used. This SiO2 surface coating layer reacts with the residual carbon content to form CO or CO2 during sintering, thereby essentially depleting it. Furthermore, although SiO2 depletes the carbon content of iron during sintering, it also improves the mechanical properties of MIM components by inhibiting grain growth, which leads to finer grains within the components.
The first Nd-Fe-B magnets were successfully sintered in 1984, although their main disadvantage is their maximum operating temperature of only 170℃. Furthermore, it is critical to avoid oxidation and carbonization during production of these components due to the binder. Indeed, to obtain magnets, the sintered parts must contain <0.1wt% of carbon, and to optimize the magnetic properties <0.08wt% is required for such magnets.
Detailed studies have been undertaken to estimate the influence of the residual carbon content on the magnetic properties as it has been demonstrated that an excess of carbon produces critical changes in the microstructure of the magnets. Indeed, magnets have a uniform cellular microstructure when the carbon content is low. However, a comparison of the cellular microstructures of magnets with different carbon contents has shown that the cell size increases with increasing carbon content and that Br and Hc are close to zero whenthe carbon content reaches a certain value (e.g., 0.43wt%inthe Sm(Co, Fe,Cu, Zr)z magnets. Furthermore, carbon could react with some components of the magnets to produce secondary phases that impede the sintering process. This is the case of the reaction between carbon and Zr, which produces a ZrC component that reduces the liquid phase of the magnet during sintering, thereby drastically impeding the densification. All these effects produce a dramatic worsening of the magnetic properties of the components, as can be seen in Fig. 14.22.
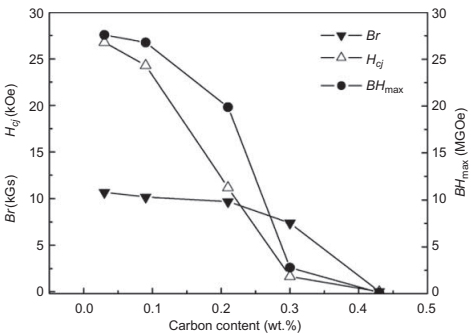
Fig. 14.22 Magnetic properties of Sm(Co, Fe, Cu, Zr)z magnets with different carbon contents
The production of other permanent magnets, such as Alnico, by MIM is yet to be optimized; therefore, such systems have received increasing interest in the last few years. Such magnets are hard and brittle, which makes them difficult to mechanize, and they have lower coercivities than SmCo or NdFeB magnets. However, the very high Curie temperature of Alnico magnets means that they can be used in systems with higher operatingtemperatures (about 540℃, compared with 180℃ for NdFeB), andthey also exhibit high remanence and very good corrosion and oxidation resistance. As is the case with the other magnets described herein, the MIM process requires the use of pure starting components and an appropriate debinding step to extract the majority of the residual carbon. The best results are obtained under a hydrogen atmosphere, although further optimization of the debinding, sintering, and thermomagnetic treatment to achieve the same magnetic properties as pressed samples is still required.
Another magnetic system, where the carbon content seems to be critical, is the nickel-base materials for applications in sensor and transducer market. For example, pure nickel alloys have increasing interest as smart materials due to their magnetoelastic properties. Recent studies have demonstrated that the MIM process is an alternative production method to obtain small parts with complex geometries as well as avoiding common defects produced by other processing techniques. Parts were sintered changing several processing parameters that have influence on magnetoelastic effects and the main results indicate that, at the optimal conditions, the estimated field-dependent elastic modulus variation, obtained in the MIM parts, was higher in comparison with variations in parts obtained by conventional processing techniques. Most of the studied parameters affecting the residual carbon after debinding and the sintering atmosphere, demonstrating that the carbon control plays a critical role in achieving the maximum field-dependent elastic modulus variation.
Control of the oxygen content during the MIM process affects the same factors as the carbon content, viz., the binder, debinding, and sintering atmosphere, therefore, both contents are usually monitored simultaneously. It is common to apply antioxidation treatments to the powders, such as an agent that covers the powder surface, thereby preventing direct contact with the atmosphere, to avoid the oxidation of magnets.
Due to their attractive properties such as low density, high strength, and good resistance to corrosion and oxidation, titanium alloys have always been important materials. However, their applications have been limited to date because of the high production costs of the raw material and the manufacturing process. Fortunately, recent research has led to a reduction in the cost of titanium products. MIM, for example, offers a low-cost alternative to otherwise expensive machining processes. Since the preliminary research in this field in the 1990s, the numerous parameters that can be varied to ensure the successful production of titanium parts have been defined. Consequently, the production of Ti by MIM is practised by several companies around the world. Despite this current production capacity, however, the MIM of titanium alloys remains a field of significant research interest due to its complexity and numerous variables.
It has been demonstrated that the key attributes of the powder are its particle size and shape, distribution, and oxygen and carbon contents. The most common MIM alloys are pure titanium (CP Ti) and Ti6Al4V, although several groups are working on other systems such as those obtained by replacing vanadium (toxic for the human body) with niobium, T6Al7Nb or Ti4Fe7Cr, Ti4Fe, Ti5Fe5Zr, Ti5Co, Ti6Al4Zr2Sn2Mo, and intermetallics such as TiAl or the shape-memory alloy NiTi.
Titanium powders are available in different morphologies, sizes, and compositions. However, the production cost of initial powders remains an important issue. Many trials are in progress on novel production routes. For example, the use oftitanium hydride powder, which has been explored since the 1990s is an attractive alternative for breaking the cost barrier. Nevertheless, only recently are the number of works describing processing conditions and properties of sintered parts increasing.
In addition to traditional thermoplastic and thermosetting binders, several types of binder have been designed in the search for easier ways to eliminate carbon residues and facilitate their decomposition. The most popular such systems are based on PW, polyethylene, PP, and on occasions, stearic acid. Indeed, binder systems based on aromatic compounds have provided some interesting results. However, although the initial solvent debinding proved to be successful, the second, thermal-debinding step was more difficult. The best option therefore appears to be a vacuum process during heating whilst at the same time leaking argon into the vacuum chamber to favor the extraction of the binder residues. Although an accurate control of the carbon content is indispensable, experience has shown that oxygen control is even more difficult in this system.
Table 14.1 Oxygen and carbon content in titanium powder obtained using different primary fabrication methods
Powder type | Median size (μm) | Oxygen (wt%) | Carbon (wt%) |
Sponge fines Hydride-dehydride (HDH) Titanium hydride Gas atomized Plasma atomized | 38 38 35 32 60 | 0.35 0.25 0.20 0.15 0.15 | 0.05 0.04 0.02 0.03 0.04 |
Control of carbon, oxygen, and nitrogen as well, is critical to success with titanium because they have substantial effects on properties (Conrad, 1981). Furthermore, the effects of these impurities are related with each other. The binder elimination has an important role on the final levels of these impurities, especially the carbon content. While the oxygen and nitrogen levels showed a major dependence on the sintering process. To gather up these interdependence, some expressions are proposed. For example, the yield strength of sintered titanium depends on density (ρ) and oxygen content (Xo):

But it is necessary to take into account that the oxygen equivalent depends on nitrogen and carbon:
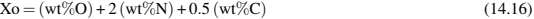
This interdependence and the high sensitivity to these impurities contents could explain why the reported properties of Ti-PIM alloys are scattered and highly variable.
Thus,the detailed study of both parameters carried out byGuo et al.inTi6Al4V system demonstrated that although an argon atmosphere results in lower residual carbon content, a vacuum atmosphere favors the reduction of the oxygen content.This finding gives someidea oftheimportance of studyingthe system as a whole. The carbon content can be measured using the combustion and infrared detectiontechnique andthe oxygen content can be addressed using an electrode furnaceininert atmosphere and equipped with both infrared and thermal conductivity detection following the ASTM method E 1409 “Determination of Oxygen and Nitrogen in Titanium and Titanium Alloys by the Inert Gas Fusion Technique.” Indeed, measurements of carbon and oxygen after different debinding times and temperatures under vacuum have shown that, whereas the carbon content was reduced to 0.095wt% after 1 h at 600°C, the oxygen contentincreased continuously during debinding (Fig. 14.23),thereby suggesting thatthe debinding process should not be extended for too long. Recent studies have described the use of PEG and PMMA-based binders that can be eliminated completely by a combination of solvent debinding in water and thermal debinding under an argon atmosphere. It is preferable to remove binders at the lowest possible temperature so that titanium does not react with the oxygen and carbon. Most of the binders decompose by about 450°C and it is observed that the impurity contents increase with higher processing temperatures. Once titanium carbides or oxides are formed, they cannot be reduced during sintering. Furthermore, the use of higher sintering temperatures leads to more contamination (mostly increased oxygen content) and reduces the ductility of the final sample. Lower temperature (in the range of 1250°C) and shorter sintering time (around 3 h) is common since containerless hot isostatic pressing is used to reach full density (a treatment between 850°C and 1100°C).
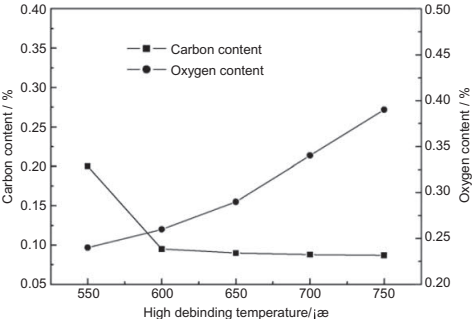
Fig. 14.23 Correlation of maximum debinding temperature during 1 h and carbon and oxygen content of the specimens during thermal vacuum debinding of Ti6Al4V. Starting carbon level of 0.056 wt% and oxygen level of 0.192 wt%.
Finally, althoughthe sintering process can be performed under argontomaintain alow carbon and oxygen content, in some cases this has been found to result in gas becoming trapped in the pores, thus resulting in low densifications. The best procedure therefore involves sintering under vacuum at a maximum temperature of between 1100℃ and 1450℃, depending on the system. These general conditions maintain the oxygen content at slightly over 0.2wt% (near to starting oxygen content of a gas atomized powder), the nitrogen content is below 0.03wt% and the carbon content at 0.04wt% orless (depending on the debinding process), and they are therefore useful for even the most demanding applications For some industrial and consumer products, a low ductility is often suitable and it is possible to allow oxygen levels up to 0.5wt%.
Table 14.2 Practical examples of material properties affected by the carbon content
Material | Examples | Debindingconditions | Sintering conditions | Carbon content | Mechanical properties or magnetic properties |
High speed steels | SKH10 | Solvent in heptane, 85℃ +thermal 600℃ in H2 N2 | 1200-1300℃ H2-N2+ heat treatment of quenching and triple tempering | 1.7% | Hardness: 70 HRC TRS: 3200MPa |
M2 | Catalytic depolymerization at 120℃ +thermal up to 700℃ in N2-H2 | 1275 1287℃, 30min in N2-H2 | 0.66%-0.77% | Hardness:57-64 HRC TRS: 1909-2315MPa | |
M2 | Thermal in vacuum up to 250℃ for 10-40 h + presintering in H2 800℃, 1 h | 1243℃, 1 h vacuum | 0.79% | Hardness: 43 HRC UTS: 800MPa | |
M2 | Thermal in argon up to 500℃ | 1250℃, 1 h, high vacuum | 0.88%-0.91% | Hardness: 620HV | |
M2 | Thermal in argon up to 450℃ | 1100 1350℃, high vacuum | 0.81%-3.2% (brown parts) | Hardness: 650HV | |
Stainless steels | 17-4PH | Thermal in H2 up to 450℃, 2 h | 1380℃, 1 h in H2 | 0.203% | TS: 980MPa |
Thermal in H2 up 600℃, 2 h | 1340℃, 1 h in H2 | 0.130% | TS: 940MPa | ||
17-4PH | Solvent in heptane 5 h 75℃ +thermal in H2 up to 1050℃ | 1250 1350℃, 1 h, vacuum or H2 | <0.1% | Hardness:25 HRC TS: 1100MPa (Af er aging treatment) Hardness: 43.5 HRC TS: 1335MPa | |
440C | Solvent in methylene dichloride 37℃,6 h +Thermal in Ar up to 600℃ + presintering up to 950℃ | 1200 1260℃, 30min in vacuum + aging treatment | 1.05% | Hardness: 57.7 HRC TS: 876M | |
Cemented Carbides | WC-8Co | Solvent in heptane, 40℃ +thermal 450℃ in H2 | 1400℃, 80min vacuum | 5.63%-5.65% | TRS: 2300 2500MPa |
WC-TiCCo | Solvent in heptane, 30℃, 2 h +thermal in vacuum | Vacuum | 6.20%-6.35% | Hardness:90-93HRA TRS: 2000-2100MPa | |
WC-8Co | Solvent +thermal in N2-H2 up to 600℃, 1.5 h | 1400℃, 1 h | 5.6%-5.7% | Hardness: 90 HRA TRS: 2500MP | |
Low-alloy steel | AISI 4600 AISI 4100 | Thermal up to 400℃ in H2- N2+ Solvent in heptane85℃ +thermal 600℃ in H2-N2 (different proportions) | 1200-1300℃ in H2-N2 (different proportions) + heat treatment of reheating + quenching +tempering | 1.2%-0.01% 0.4%-0.5% | TS: 1400MPa Hardness: 33 HRC Elongation: 9% |
AISI 4605 | Solvent in heptane +thermal debinding 500℃ | 1140-1360℃ in N2 | 0.41% | YS:400MPa After heat treatment YS: 1300MPa | |
AISI 4605 | Solvent in heptane +thermal debinding 500℃ | 1140-1360℃ in N2 | 0.75% | YS:450MPa After heat treatment YS: 1470MPa | |
Titanium alloy | CP-Ti Grade 2 | Solvent in water, 50℃, 6 h +thermal debinding in argon up to 440℃ | 1300℃ in argon, 3 h | 0.04% | TS: 483MPa Elongation: 21% |
CP-Ti Grade 4 | Solvent in heptane+ thermal debinding and dehydrogenation under argon | 1200℃ in argon | 0.065% | YS: 519MPa TS: 666MPa Elongation: 15% | |
Ti6Al4V | Solvent in heptane, 80℃, 5 h | 1250-1280℃ in vacuum | 0.073% | EM: 122 GPa YS: 865MPa UTS: 955MPa Elongation: 12% | |
Ti6Al4V | Solvent in heptane, 40℃ 20 h | 1250℃, in vacuum, 2 h | 0.05% | YS: 700MPa TS: 800MPa Elongation: 15% | |
Magnets | Alnico 8 | Solvent in acetone, 45℃, 18 h +Thermal in H2 | Sintering in H2 1300 1325℃ +Thermoma netic | 0.183% | Br ¼0.772T Hcb =85.19 kA/m (BxH)max =22.95 kJ/ m3 |
Nd-Fe-B | Thermal 300℃, 30min H2+ 30min vacuum | 1080 1120℃, 4 h in vacuum + annealed in vacuum 500℃, 2 h | 610-790 ppm | Br =1.268T iHc =0.68-1.10 MA/m (BxH)max =306-287 kJ/m3 |
Contact: Cindy Wang
Phone: +86 19916725892
Tel: 0512-55128901
Email: [email protected]
Add: No.6 Huxiang Road, Kunshan development Zone, JiangsuShanghai Branch: No. 398 Guiyang Rd, Yangpu District, Shanghai, China