It is interesting to note that there are only a few current applications of powder metallurgy (PM) to precious metals but commercialized PM production began with precious metals, specifically platinum (Roll, 1984). Platinum was available as sponge (agglomerated powder) from refining or fine particles from mining. Melting platinum was very difficult and mainly unsuccessful. Consolidating platinum particles (powder) by applying pressure established enough particle-to-particle contact area and provided adequate thermal conductivity so that the compact could be further densified by heating (sintering) and working. Wollaston is credited in using "PM" in the first scaled production of malleable platinum in the early 19th century. The use of the Wollaston method became unnecessary as methods to bulk melt platinum were developed in the mid-19th century, ceasing further use and development of precious metal PM. That is not to say there are not other commercial uses for precious metal powder, they just lie outside of traditional PM processing. For example,
Structural solders/brazes in paste form: Solder and braze alloys (Ag-, Au-, and Pd-based) are available in paste form to facilitate their placement to braze joints. A metal powder of the solder or braze alloy is mixed with an organic vehicle (paste). The paste volatizes upon heating and the powder melts and fuses the joint.
Electronic solders and thick-film applications: Similar to solder and braze pastes, precious metal (gold and silver) powder (usually chemically precipitated) is mixed with an organic to form an "ink". This ink is applied to an electronic substrate by screen lithography to form conductive paths or to pot electronic components. The substrate is heated causing the organic to volatize and the powder to sinter into a coherent conductive path. There are some inks that do not require the removal of the organic carrier to form conductive paths.
Dental: The most common application for precious metal powder in dentistry is in amalgam restorations (fillings). Alloy powder of the silver-copper eutectic and an alloy powder of silvercopper-tin are mixed with mercury and applied to the prepared tooth cavity. The mercury subsequently diffuses into the powder and forms a solidified amalgam. Other dental applications include the Captek process (Product of Argen Corporation, n.d.); a gold alloy powder mixed with a polymer and applied as a sheet to a porcelain substrate and subsequently fired and similar applications with palladium alloys (Product of Nobil-Metal S.p.A, n.d.).
Electrical: Electrical contacts and motor brushes are made from silver-graphite, silvertungsten, silver-molybdenum, silver-(Cd or Sn) oxide, or silver-nickel composites. Silver is used as the current carrier and the other materials add strength, arc resistance, and lubricity (in the case of graphite in motor brushes) to the composite. Press and sinter, hot press, and extrusion are all used in the production of precious metal electrical components.
PMC (Precious Metal Clay): This is a relatively recent application. Precious metal powder (usually pure Ag, Au, or Pt), usually precipitated but can also be fine atomized powder, is mixed with an organic binder to yield a clay-like product. This is molded by hand to a part, which is subsequently fired-burning out the binder and sintering the powder. High densities are not achieved limiting this process to non-critical cosmetic items (jewelry).
Additive Manufacturing (AM): There is substantial interest in precious metal parts made by additive manufacturing, specifically via laser powder bed fusion. Most of the activity involves jewelry and watch cases, both of which are design-driven. EOS (www.EOS. info/press/customer_case_studies/glittering_prospects, n.d.), among other AM equipment manufacturers, make small chamber systems to minimize the high cost of filling a large conventionally sized build chamber. The majority of the interest is in Europe at this time.
As shown, there are many uses for precious metal powder but most are outside of what is considered traditional PM technologies. MIM for conventional materials, such as stainless steels, was able to develop quickly by using the extensive resources and knowledge base of press and sinter PM. There is no similar knowledge base or supply for precious metals. This has hindered the adoption of precious metal PM and MIM and only recently has there been commercial use of MIM in precious metal part production.
Since PM is not common in precious metal parts manufacturing, Design for Manufacturing (DFM) guidelines for precious metal PM have not been established. Thus, in order to ascertain potential applications and opportunities, one must review the current processes and parts made from precious metals.
Parts made from precious metals include the following:
Electrical/electronic contacts
Medical: fiduciary markers, electrodes, embolization coils
Coins/medallions
Watch cases, watch parts
Jewelry
The manufacturing methods to process these precious metal items include the following:
Investment casting
Deformation processes (rolling, extrusion, drawing, stamping, forging/striking/coining)
Machining (lathe, mill)
AM (Additive Manufacturing)
Electroforming, electrophoresis
In the following general guidelines for MIM applications, MIM competes well with investment casting and discrete machining. Tube, sheet, and wire products are generally not complimentary to PM processes, nor are electroformed or AM parts. The parts that are commonly made by investment and discrete machining and also manufactured in quantities that would justify the cost of implementing MIM would primarily entail jewelry parts and watch parts. Precious metal watches are traditionally forged from wrought alloy to provide strength so these may not be good MIM candidates. Thus, for the most part, the precious metal manufacturing sector with the most potential for MIM are jewelry and jewelry parts (clasps, ferrules, etc. AKA "findings") that would be normally be investment cast. Further discussion will address the jewelry sector.
There are three driving forces that justify the use of any PM process over other manufacturing technologies:
PM enables a lower manufacturing cost.
PM is the only possible way to make the part.
PM produces a material or part of higher quality than other processes.
The vast majority of PM applications follow #1. Far fewer applications follow the other two reasons. Cemented carbides are a PM-only process as these composite alloys cannot be cast. PM (hot isostatic pressing (HIP)/extrude/forge) of nickel-based superalloys eliminates the solidification segregation of casting, producing a superior material. Certainly, there are other examples apart from these exceptions but the majority of PM applications are justified from cost savings.
The use of MIM in precious metals must follow the same rules. Precious metal MIM either has to have a lower unit manufacturing cost (at equivalent quality levels), it must offer attributes that cannot be done via conventional jewelry manufacturing processes, or it must produce a superior product.
Investment casting of jewelry is fragmented and diverse. There are some items that are unique and made in low production volumes. Other more universal parts, such as clasps, which can be used on a multitude of different products, are sometimes produced in large volumes (>50,000 parts per year).
If one considers a dental bracket, a jewelry finding is very similar in size and geometric complexity. Thus, it follows that MIM would offer a cost advantage over investment casting, as it did for dental brackets. However, unit cost is only part of the cost equation with precious metals.
Much of jewelry production is essentially Just in Time (JIT). That is, parts are only cast when there is an order. Precious metal parts are rarely inventoried due to the high cost of metal. For example, the cost per kg of bulk 18 kt gold, 14 kt gold, and sterling silver are currently $32,000, $24,700, and $500, respectively. The bulk of the precious metal inventory is not owned by the jewelry manufacturer. Rather, it is leased from banks and repositories. The manufacturer leases the material to fulfill their production requirements and pays back the lease when they are paid for the product (no long-term payments in jewelry). Thus, time is of the essence for throughput rates in manufacturing.
In casting, the investment casting cores are made of a polymer and these can be inventoried at very little cost. When an order for a part is made, the inventoried core is added to a production casting tree. The time between receiving the metal, casting it, retrieving it, and finishing it can be less than a day, keeping the lease costs to a minimum. However, for MIM, there is additional time for the following: (1) feedstock preparation from the powder, (2) debinding of the part, and (3) sintering of the part (molding and finishing are assumed to be of the same time scale as for an investment casting). The MIM operation adds at least 24 h to the time to produce a part. In addition, powder production will add another 24-48 h to the time scale. This will add significantly to the cost of materials in the form of lease costs. However, this lease cost will be substantially different between silver and gold alloys. The cost of silver is significantly lower than gold, platinum, or palladium so the cost associated with time-inprocess may not be an issue with silver.
Part of the JIT processing implies a high degree of flexibility; parts are only made when ordered. Consequently, for MIM to allow this degree of flexibility, individual parts would have to be able to be molded on demand. This would only be possible with small easily changeable molds. While this is possible it is not typically practiced. Most of MIM tooling is designed for long production cycles. Thus, at this time any application of MIM for jewelry would have to be for large volume production runs.
MIM does offer some cost advantages over jewelry investment casting. MIM is inherently a lower labor cost operation. The lower unit labor cost is a result of a reduction in labor per unit part and also a lower labor cost due to lower skill sets required. Investment casting entails more steps at mid to high level of labor skill sets: assembling the casting tree, casting, and removing the part from the cast tree. Removing the cast part from the tree leaves a sheared area that requires more finishing compared to the gate area of a MIM part. Fig. 25.1 is a simplified schematic comparing a typical investment casting operation with a MIM operation. The MIM operation has fewer high relative cost unit operations, and fewer unit operations overall.
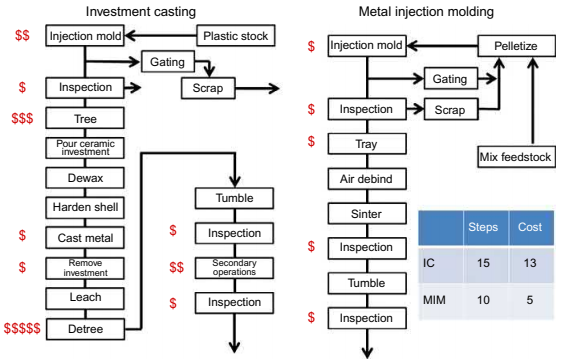
Fig. 25.1 A schematic of unit operations and relative costs for investment casting vs. MIM. Powder injection molding (PIM) technology: overview and applications to the jewelry industry. In Proceedings of the 7th Santa Fe symposium in jewelry manufacturing technology.
In investment casting, the bulk of the tree and sprues, etc. can only be reused after cleaning and remelting. The recycle loop for MIM sprues, gates, runners, etc. is smaller as they can be granulated and put back into the molding feedstock; which is a lower cost operation than cleaning and remelting. In addition, there is less material per part as trees are not used in MIM to fill the mold cavity. Fig. 25.2 shows an investment cast tree of jewelry items. It can be seen that the volume of the tree is typically much greater than the jewelry parts being filled. Although reusing this excess can be as simple as cleaning and adding it to virgin material, the ratio of sprue to part is usually much >1 so used material to be recycled is generated faster than product. This implies that much more material needs to be inventoried for casting production than for MIM production.

Fig. 25.2 Investment cast trees showing the relative volume of parts to feed and support structure
There are many economic considerations to be taken into account to make a case for MIM on cost alone. Labor savings are still considered the primary driver in MIM applications and for this reason MIM for jewelry, although developed in the US, was investigated in Europe prior to US applications. Fig. 25.3 contains examples of iconic European jewelry items from an early R&D study with MIM. Certainly, there are instances where MIM is justified, as there are several cases where MIM is used in production for sterling silver jewelry items.

Fig. 25.3 Two as-sintered iconic jewelry articles MIM'd in 18 karat gold (coin for scale)
With respect to material properties, MIM will produce a microstructure that has a finer grain structure with an attendant improvement in mechanical properties and a reduction in chemical segregation with respect to investment casting. This can translate to improved tarnish resistance which is an important attribute in jewelry. In addition, the finer grained microstructure produces a better polish than coarse grained material.
MIM can offer some design features that are difficult to achieve with investment casting. Sinter-bonding can be used to make complex parts too difficult to mold or cast. Fig. 25.4 shows some hollow jewelry parts made by joining two parts during MIM processing. Molding over a consumable insert can also produce a hollow part as shown in Fig. 25.5. The insert dissolves during debinding.

Fig. 25.4 Sinter-bonded parts to make a hollow ring. Top: Green sterling silver hollow half ring.
Bottom: Sintered and sectioned ring. Right: Sintered and semipolished ring.

Fig. 25.5 Hollow sterling silver parts made by incorporating a consumable insert. Left: Green sterling silver ring with insert. Top: Soluble insert. Right: Sintered sterling silver ring (coin for scale).
Flat parts or parts with abrupt changes in cross-sectional thickness are problematic in investment casting. Thin flat parts, such as medallions, are difficult to feed during casting and abrupt thickness changes can produce shrinkage porosity at the intersection (Tyler Teague, 2018). MIM of thin flat parts is not an issue and can be readily done. Fig. 25.6 shows two medallions made by MIM that would be very challenging to investment cast.

Fig. 25.6 Sintered 18 karat gold parts made by MIM. Left: HJE medallion (with gate) with a thickness of 1.5mm. Right: Sports team medallion with a thickness of 1mm and a loop thickness of 0.5mm. Center: Dental implant test part. The medallions are as-sintered. The dental implant part is polished (coin for scale).
The porosity level of a jewelry part can be controlled through MIM processing.Porosity is not generally a positive attribute as it often causes surface defects or part failures for investment cast parts, where it usually exists as macro-porosity. However, in MIM, porosity exists as isolated micro-porosity and the amount, size, and distribution of the porosity can be controlled. The end goal of the jewelry part is to produce a surface of acceptable quality. Since most commodity jewelry items are sold on a per piece basis, having a part with a few weight percent porosity would mean a substantial saving in material and higher profits for the manufacturer. MIM development work with sterling silver found that up to 6% porosity could be incorporated and produce an acceptable surface finish for items such as signet rings and up to 3% porosity could be tolerated for higher-level jewelry pieces.
One of the key impediments to the adoption of precious metal MIM was the limited sources of MIM-grade precious metal powders. The transition from cast or machined dental brackets to MIM was straightforward as MIM grade stainless powder was readily available. The sources of gas atomized precious metal powders for use in precious metal solders and brazes cannot produce powder fine enough for MIM use. Atomization technology and equipment is available for making MIM-grade precious metal powder but until recently it was not common. The recent interest in powder bed additive manufacturing for jewelry has resulted in numerous new facilities with atomization systems dedicated to the manufacture of precious metal powders. This has generated renewed interest in precious metal MIM simply because the powder is available.
Other barriers to the adoption of MIM in precious metals include the cost of the MIM equipment and the availability of the technology knowledge base. As MIM has grown for conventional materials, equipment specifically designed for MIM has become more available. The MIM technology (binder formulations, processing knowledge, etc.) has also become more available via published articles, trade conferences, and a significant and mobile MIM engineering work force.
If precious metal MIM will progress, will a conventional MIM facility become a toll processor for a jewelry company or design house or will a jewelry facility integrate the MIM process into their operation? It is most likely that precious metal MIM will occur within the jewelry manufacturer (or precious metal manufacture) primarily because of the cost of the material and the security needed in the facility.
Jewelry made of precious metals must meet industry requirements of established jewelry alloys in order to be hallmarked. Sterling silver is probably the most common jewelry alloy. Sterling silver has a minimum of 92.5% silver with the balance being copper or predominantly copper. Thus, sterling silver jewelry is hallmarked with a symbol that includes the numerals "925" meaning 925 parts silver per thousand. Pure silver (AKA "fine" silver) is marked as "999" meaning a minimum of 999 parts silver per thousand. Coin silver has a minimum of 90% silver and is thus marked "900".
Gold jewelry alloys are based on the "karat" system. Pure gold is 24 karat, meaning 24 parts gold per 24. In the US, the most common karat alloys are 18 (18/24 or 75%), 14 (14/24 or 58.3%), and 10 (10/24 or 41.7%) and these numbers will be part of the hallmark for these alloys. Gold is also available as 22, 20, and 12 karat as well. The karat denotes the minimum gold content and the balance can conceivably be anything else. However, the alloying elements are chosen for compatibility to produce alloys that are processible and have properties needed for jewelry (strength, ductility, tarnish resistance). The simplest gold alloy is a ternary of gold, silver, and copper. Different combinations of these elements will yield alloy colors of white, yellow, green, pink, and red. Fig. 25.7 is a ternary diagram that maps out the various alloys with respect to color and karatage. White golds often replace the silver with nickel, zinc, or palladium. Nickel is being universally eliminated due to skin sensitivity, palladium is currently too costly, leaving zinc as a common alloying addition. Zinc is considered a requirement in 14, 12, 10, and 9 karat alloys as it richens the color. These alloys without zinc do not have as an acceptable a color. There are other trace elements that are often added to act as grain refiners and deoxidants as well.
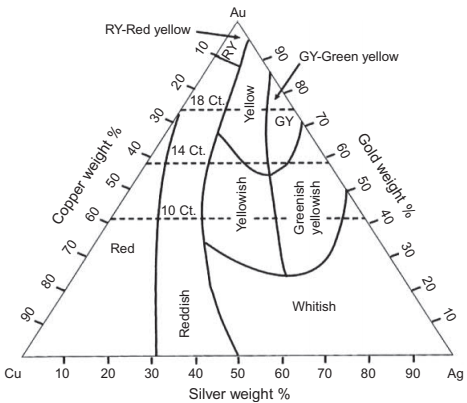
Fig. 25.7 Au-Ag-Cu ternary phase diagram showing color regimes
Platinum and palladium alloys are also marked as silver in parts per thousand, the most common alloys being 950 and 900, although 850 is also sometimes available. As with the other jewelry alloys, the noble metal content is called out and the remainder can be anything. Platinum is commonly alloyed with ruthenium, iridium, or cobalt. Palladium is commonly alloyed with ruthenium, gallium, and sometimes copper.
In order for any of these precious metal alloys to be a candidate for MIM there are two obvious basic requirements: (1) the alloy is able to be atomized and (2) the atomized powder must be able to be sintered. There are challenges to each of these that are alloy-specific.
Silver and gold alloys are readily atomized. All are relatively easy to melt as the liquidus temperatures are all modest with respect to stainless steels and the alloys are not very reactive. Conventional close-coupled inert gas atomization has been used to make MIM grade silver and gold alloys. There is also potential for atomizing these alloys with high pressure water atomization as is done for producing very fine stainless steel MIM powder.
As for sintering, most silver and gold alloys for jewelry will sinter. The primary issues come from the alloying additions that may inhibit sintering. For example, in karat gold alloys that contain zinc, the zinc oxide that forms during the atomization, handling, or in the MIM processing (specifically the debinding step) will not reduce at temperatures below the solidus temperatures. This will impede sintering. For gold alloys, it is recommended to use alloys of the gold-silver-copper ternary system.
Casting alloys often contain deoxidizers and grain refiners. Neither is necessary if the alloy is to be atomized for MIM processing.
Platinum and palladium are not as straightforward to atomize due to their very high liquidus temperatures. The temperatures needed for atomization are much higher than those used for casting, which can be challenging for the refractories used to contain and control the melt during atomization. For example, many of the platinum jewelry alloys have liquidus temperatures approaching 1800℃. Casting may only require 50℃ superheat but close-coupled atomization for producing MIM-grade powder may require 100℃ to 200℃ superheat and for sustained times, which is above the use rating of most refractories. Nevertheless, there is at least one commercial source for atomized platinum alloy powders using gas atomization. Recent developments in crucible-less atomization (EIGA, plasma wire, and plasma powder spheroidization) may have potential to make MIM-grade platinum alloy powder. Palladium jewelry alloys require atomization temperatures around 1700℃, making them less challenging.
Precious metals are also available as "sponge," the precipitated and agglomerated powder product that comes from refining. These powders are only available in the elemental metal, not alloys. While it is possible to make precious metal alloys via elemental mixing of powders, these precipitated powders are not optimum for MIM due to their high surface area and attendant low solids loading capabilities in the MIM feedstocks, which create difficulties in molding.
MIM processing of silver and gold alloys is straight forward and very similar to MIM processing of conventional materials. The two primary differences are in binder formulation and tooling design.
With respect to binder formulation, of primary importance are (1) the binder must be removed at a relatively low temperature and (2) debinding must not leave any carbonaceous residue. A binder for silver and gold jewelry alloys must accommodate the alloys' low sintering temperatures and zero tolerance for carbon. Sintering of these alloys can initiate as low as 300℃. It is necessary that the binder be completely eliminated at these low temperatures or else residual binders will be trapped in the part. Related to this is that carbon is essentially insoluble in silver and gold jewelry alloys. Unlike iron-based alloys, where residual carbon from the binder can be dissolved and diffused through the matrix to be subsequently removed in a low carbon potential sintering atmosphere, any residual carbon in a jewelry alloy compact will be trapped within the inter-powder microstructure with essentially no mechanism for its removal. This could impede sintering to full or usable density and the resulting pores or entrapped carbon could affect the cosmetics of the part. Wax-polymer binders suitable for copper MIM will be satisfactory for silver and gold jewelry alloys. Typical solids loading for gold and silver MIM feedstock can be between 57% and 67%, depending on the particle size distribution of the powder.
Thermal debinding can be done in air as long as the alloy does not contain alloying additions whose oxide cannot be reduced during the sintering cycle. In Section 25.3, zinc was stated to be necessary to provide acceptable colors in 14 karat and lower alloys. Zinc oxides will be problematic as the oxide does not reduce until above the sintering temperature range of these alloys. The copper in the silver or gold alloy will oxidize during thermal debinding but copper oxides will be reduced in the subsequent sintering in a reducing atmosphere.
Silver and gold alloys have higher-thermal conductivities than conventional materials. This enhanced heat transfer translates to the powder as well so the feedstock will have a higher-thermal conductivity and can better transfer heat to the tooling leading to faster cooling and solidification. This can be rectified by increasing the crosssectional area of the runners and sprues and by using warm tooling or hot sprues if found necessary.
Sintering of these alloys is also straight forward. It has been found that a reducing atmosphere of nitrogen and hydrogen in almost any ratio is satisfactory for sintering. The sintering temperatures are alloy-dependent but tend to be within 100 degrees C of the solidus temperature.
PM with platinum and palladium alloys is largely unrealized. Press and sinter PM for discrete jewelry articles was proven possible with precipitated powders and water atomized powder but MIM was not attempted as those powders were not appropriate (too coarse). Sources of gas atomized platinum powder have only recently become available.
One of the issues with platinum and palladium MIM is that both of these metals are catalytic to hydrocarbons. It has been established that care must be taken in preparing thick-film pastes that use organic binders to avoid fires. However, thick-film pastes use precipitated powders, which have a much greater specific surface area than atomized powders so the catalytic action may be suppressed. This is currently an area of research.
Carbon is highly soluble in both platinum and palladium. When solubilized in the melt the carbon is ejected during solidification and forms graphite plates, much like the graphite in gray cast iron, and this negatively affects the mechanical properties of platinum and palladium alloys. Carbon that goes into solution in the solid phase during sintering may not behave in the same manner. This should not be an issue if the binder is removed entirely during debinding. The use of an oxidizing thermal debinding environment will be necessary.
Sintering of platinum and palladium powders should be achievable. Pure platinum and palladium could be sintered in air, as done with the thick-film pastes. However, jewelry alloys may contain elements that will require an inert or reducing atmosphere. Ruthenium is commonly added to platinum and ruthenium will form an oxide that is volatile within the sintering temperature range so oxidation should be avoided. Copper, cobalt, gallium, and germanium are also common alloying additions. All of these will oxidize therefore requiring a protective sintering environment.
Hydrogen is very soluble in palladium and, to a lesser extent in platinum and will cause an appreciable lattice dilation. However, this is reversible upon cooling. If hydrogen is necessary to reduce alloy element oxides, it may be feasible to use a partial pressure of hydrogen to reduce the oxides and then finish the sintering under an inert gas atmosphere alone. This is an area that needs further development.
The driving force for MIM platinum or MIM palladium may be greater than that for gold and silver alloys. Gold and silver are readily castable whereas platinum and palladium are difficult to cast. If MIM were proven for platinum and palladium alloys, this would present many opportunities in jewelry manufacturing.
In jewelry manufacturing, a prime goal is to achieve a high quality reflective surface finish. Most MIM products are above 95% density, with many approaching 99%. The residual porosity is very fine and evenly distributed in the microstructure. However, a metallographic examination will reveal this porosity even without magnification and it appears as a slightly matte finish or haze. This will not be acceptable for jewelry. That said, metallographic preparation is not applicable to jewelry. Jewelry finishing methods tend to work the surface, which produces a burnished surface and may conceal some surface defects. Thus, a finishing method that works or smears the surface is required to conceal the porosity. Involute surfaces respond well to tumbling with metal media (needles). Flat surfaces may require surface peening via tumbling prior to flat face polishing.
Contact: Cindy Wang
Phone: +86 19916725892
Tel: 0512-55128901
Email: [email protected]
Add: No.6 Huxiang Road, Kunshan development Zone, JiangsuShanghai Branch: No. 398 Guiyang Rd, Yangpu District, Shanghai, China