MIM of titanium and titanium alloys(19.1-19.4)
Table of Contents
19.1 Introduction
Since around 1940, when William Justin Kroll introduced the first economic and industrial process to gain metallic titanium from the ore, titanium has been used for industrial and commercial applications at a continuously progressing rate. However, compared to steel, the annually produced mass is still rather small and applications are mainly limited to five fields: the off shore industry; chemical industry; aerospace and automotive industries; medical devices and implants; and luxury goods. The listed application areas are directly linked to the specific characteristics of titanium, which are the very high strength to density ratio, the excellent corrosion resistance against salt water and most chemicals, the high biocompatibility and the warm and optically attractive surface of the material. Depending on the demands with respect to corrosion resistance, mechanical properties and also costs, either pure titanium or an alloyed material is used. High costs of raw material and processing are the main reasons for the limitation to applications where the specific properties of titanium and titanium alloys are so beneficial that higher prices can be tolerated. Today, the light-weight properties and biocompatibility in particular are features generating high levels of interest; the usage of titanium-based materials could probably be much higher if the price of the product was lower.
Against this background, metal injection molding (MIM) appears to be ideal for the processing of titanium, as typical advantages are high material utilization and low production costs in large-quantity manufacturing. Machining of titanium is rather expensive owing to high tool costs and low processing speed. Thus, the typical geometry of machined titanium parts is rather simple and is not optimized with regard to functionality. On the contrary, even highly complex components can be manufactured by MIM without necessarily increasing costs. AM techniques like selective laser melting (SLM) or electron beam melting (EBM) are alternative technologies, but the costs are still high and for high production volumes, MIM has significant advantages. However, even if today MIM is an established technology processing of titanium materials remains relatively rare, even if a continuously increasing interest is noticeable. In this chapter, the specific challenges of titanium MIM and methods to overcome these challenges are presented. Furthermore, the current status of MIM of titanium is reported with respect to available feedstocks and mechanical properties. Finally, current research activities are explored, with a view to prospects for the future.
Table 19.1 Maximum oxygen level and tensile properties according to ASTM B348-02
| Max. oxygen content (wt%) | Min. YS (MPa) | Min. UTS (MPa) | Min. εf (%) |
Ti Grade 1 | 0.18 | 170 | 240 | 24 |
Ti Grade 2 | 0.25 | 275 | 345 | 20 |
Ti Grade 3 | 0.35 | 380 | 450 | 18 |
Ti Grade 4 | 0.40 | 483 | 550 | 15 |
Ti-6AI-4V Grade 5 | 0.20 | 828 | 895 | 10 |
Ti-6AI-4V Grade 23 | 0.13 | 759 | 828 | 10 |
19.2 Challenges of MIM of titanium
19.2.1 Interstitial elements
The most important feature of titanium that represents a challenge forpowder metallurgy (PM) is the high affinity to interstitial elements like oxygen, nitrogen, carbon and hydrogen. The fact that titanium is used in the vacuum technique as a getter material for purification of the atmosphere from oxygen clearly shows the problem that arises when fine powders have to be handled. According to the binary-phase diagram for Ti-O, titanium is able to absorb 13wt% oxygen on interstitial lattice sites at elevated temperatures. The great affinity to interstitial elements is combined with a strong influence on the mechanical properties, even at low contents in the range of 0.1-0.4wt%. For illustration, Table 19.1 shows the values for the maximum content of oxygen according to ASTM standard B348-02 for wrought bars and billets and its influence on tensile properties.
Even if the standard also regulates maximum values for iron, nitrogen, and carbon content, oxygen is the most important element with regard to its influence on mechanical properties, because it is picked up preferentially. The values given in the table are just minimum limits. However, it can be estimated from these numbers that in case of pure titanium an increase by just 0.22 wt% oxygen from grade 1 to grade 4 effects more than a doubling of the tensile strength. On the other hand, the ductility is reduced strongly and this is essentially the reason why the pickup of interstitial elements during MIM processing has to be minimized. It is rather easy to obtain a high strength in a MIM titanium component just by increasing the oxygen content, but the provision of good ductility is a challenge. In the case of titanium alloys like Ti-6Al-4V, the oxygen limits are even smaller (see Table 19.1) than those of pure titanium grade 2. There is also a standard for PM products made from titanium alloy powders (ASTM B817) allowing an oxygen content of 0.3 wt% for Ti-6Al-4V. However, this standard is commonly not applied for MIM components, not even for comparison.
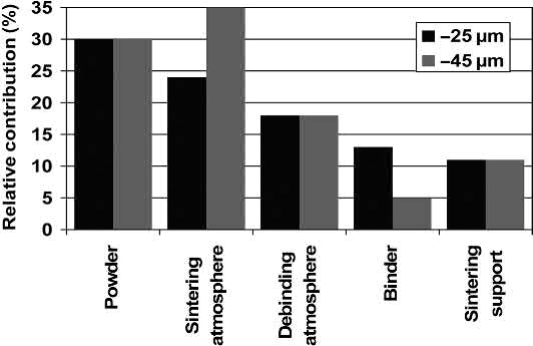
Fig. 19.1 Relative contribution of MIM processing parameters to final oxygen content of the sintered part in relation to dependence on maximum powder particle diameter. Interstitals sources and control in titanium P/M processes.
From the point of view of solution hardening oxygen, nitrogen, and carbon have the same effect, but with different potency. Therefore, it is sensible to combine these elements in the form of an oxygen equivalent Oeq. A commonly used equation is

where cO, cN, and cC represent the concentrations of oxygen, nitrogen, and carbon, respectively. In the literature, the coefficient of cC differs somewhat between 0.5, 0.66, and 0.75. In addition, Eq. (19.1) is given in atom percentage, but it is often applied in the literature by using weight percentage. However, in practice this difference does not really matter because the amounts of carbon and nitrogen are rather low compared to oxygen and the atomic weights of O, N, and C are comparable. Even if the effect of nitrogen is twice as high as that of oxygen, again in practice the latter remains the most important interstitial element. This is mainly due to its high diffusivity, the high solubility and the fact that the oxide layer spontaneously formed at the surface impedes penetration by other elements. Experience shows that during MIM processing the pickup of nitrogen is usually no problem. Even hydrogen commonly need not be taken into account because sintering of titanium is usually done under high vacuum at high temperatures. Under this condition, possible hydrogen contents are drawn out from the material. On the other hand, carbon has to be considered, because it is present in all binders and during high-temperature steps like thermal debinding it can be absorbed. Therefore, it is more sensible to use Eq. (19.1) instead of just considering the oxygen content when discussing results from mechanical tests performed on MIM-processed materials.
A comprehensive study about the sources of interstitials, especially oxygen, during MIM processing. As shown in Fig. 19.1, several sources exist, starting with the powder and ending with the sintering support which is commonly made from ceramic materials like Y2O3 or ZrO2. The ratio between these sources depends slightly on the powder particle size, but the overall pickup is nearly the same. The study shows that storing and handling of the powder is not critical as long as the temperature is below 200℃. Above 400℃ rapid oxidation starts. Even the uptake from the binder is tolerable, if the right binder components are used and debinding is performed adequately, as shown in the next sections. Most critical are the interstitial content of the powder and the sintering atmosphere, which has to be as free as possible from oxygen.
19.2.2 Powder and feedstock
Today, the availability of commercial titanium-based MIM feedstock appears to be rather poor compared to stainless steel or cobalt (Co)-based materials. For many years, only pure titanium feedstock of specification grade 4 was commercially available. Components made from this material show sufficient strength but the ductility does not fulfill the ASTM requirements. Today, a variety of Ti-6Al-4V feedstocks can be bought on the commercial market, resulting in components with much higher strength and fracture elongation of >10% if processed adequately. However, it is common practice, even for commercial MIM suppliers, to manufacture the feedstock in-house by blending powder and binder components. Production of feedstock in-house is not a serious problem and provides great freedom with regard to powders and alloys used.
Powder
The available powders can be divided very roughly into pure and expensive and comparably unclean and of lower cost. In this context, "pure" relates especially to the oxygen and carbon content, but also to residuals from the specific production process such as Cl, Ca, Na, or Mg. Furthermore, these powders differ in geometry: the expensive ones are more spherical, the lower cost powders are typically irregular shaped and often agglomerations are found. Current techniques for the production of more expensive powders are inert gas atomization, plasma atomization, and plasma rotating electrode processing. Because alloys can be used as starting materials, prealloyed powder can also be made by these techniques. Lower cost powders are made by mechanical milling of sponge, scrap or ingots, for example as done in the hydride-dehydride (HDH) process; the raw material is loaded with hydrogen effecting a drastic embrittlement of the material. Thus, it can be milled easily and the hydrogen is extracted again by thermal treatment under vacuum. It is also possible to use scrap or ingot as raw material. Thus, different purities of HDH powder and also alloys are available. There is much research on reduction of the titanium oxide without Kroll process aiming at lower cost powder. Especially, powder production directly from the oxide is of great interest. However, at the moment HDH powders are mostly used as an economic alternative, if the properties of the sintered parts are sufficient. Generally, HDH powders show a relatively high content of oxygen. This is because passivation steps have to be included in the processing to avoid self-ignition and burning of the powder. In addition, rather high temperatures are involved which are sufficient to effect sintering between the particles. More or less pronounced agglomeration is therefore typical. A further process is able to reduce the oxygen content after powder production. Liquid Ca is utilized in order to reduce the titanium to levels fulfilling the corresponding ASTM standards. However, this additional process will increase the powder costs and only the future will reveal whether this method will provide an attractive powder for the market.
Using HDH powder could even assist in keeping the oxygen level low, if the still hydrided powder is used for sintering. The atomic hydrogen escapes during sintering and reacts with possible oxygen in binder, powder and furnace atmosphere. Furthermore, sintering of TiH2-powders leads usually to high densities.
Choosing the right powder for MIM means taking into account the required mechanical properties, technical issues like powder flowability, component geometry and costs. Spherical powders produce the lowest viscosity feedstock system; thus, binder content can be kept low and sintering activity is high. Combined with high purity, using these powders usually results in the best mechanical properties. However, up to now spherical powders are relatively expensive. Fortunately, the growing application of Additive Manufacturing (AM) technologies has led to significantly better availability of spherical powders along with decreasing prices. Powder-bed-based AM techniques like SLM and EBM rely on excellent spherical powders.
If the requirements of strength and ductility are not that high, HDH titanium powder may be sufficient. In addition, irregular-shaped powders give higher green part strength. It is also possible to mix both kinds of powders to reduce the material costs, as will be shown later. Even alloys can be produced by blended elemental technique, if the specific prealloyed powder is not available. Again it is possible, for example, to mix lower cost HDH titanium powder with more expensive prealloyed powder to gain the final composition in the sintered part. Irregular shaped powders can also be spheroidized by plasma techniques if required and economically sensible.
Because of the different production techniques compared to steel powder and because of the contamination problem, which is more important when finer powder is used, for MIM of titanium commonly the size fraction <45μm is utilized. Accordingly, it is significantly coarser than the usual stainless steel powder. One disadvantage is the greater tendency for separation of binder and powder during injection molding. On the other hand, even with these powders, wall thicknesses of the sintered part below 0.2mm can be realized without significant problems.
Binder
Several binder systems exist which are suitable to be used combined with titanium powder. Basically, the composition should provide the possibility for debinding in two steps, typically by a solvent and a thermal process; however, a binder for catalytic debinding is also available. In the latter case, for example polyacetate can be used as one component. For solvent debinding, the binder often comprises paraffin wax, for water debinding polyethylene glycol (PEG). Also, the use of naphthalene is possible, which evaporates slowly from the green part. The second component decomposes by thermal treatment prior to sintering, usually at temperatures above 400℃. Thus, oxidation and carbonization are possible risks at this stage. Therefore, the second component should contain as little oxygen as possible and should decompose in the right temperature range. Generally, the thermal degradation temperature should be as low as possible, because the higher this temperature is, the higher is the risk for contamination of titanium by oxygen and carbon. On the other hand, first sintering has to start before the binder has escaped completely, because of mechanical stability. Degradation temperatures between 400℃ and 500℃ are quite suitable. First bonding of titanium powder particles starts at about 600℃ or less.
In practice, polyethylene or polypropylene, even mixed or as copolymer with ethylene vinyl acetate, are used with good success. The ratio of first and second binder component depends on the required green part strength and the powder used, and also on the acceptable contamination level after sintering. A higher amount of the second component means basically an increase in oxygen and carbon content. Between 20 wt% and 40 wt% of the second component is typically a good value. A third typical additive is stearic acid, working as a surface active agent improving the wettability of the powder and influencing viscosity and mold release. There is a wide range of percentage from 1% to 12% used.
19.2.3 Facilities
There is nothing completely different with regard to the MIM equipment when titanium is being processed. However, the contamination problem has to be considered in the whole production chain; consequently, the precise specification of the devices is influenced. If the feedstock is made in-house, kneading under a protective atmosphere could be considered, if possible. Any device suitable for MIM of stainless steel can be used, as an injection molding machine. The mass temperature during injection ranges typically between 120℃ and 180℃; the mold is heated to temperatures between 40℃ and 60℃. The precise parameters depend on the feedstock and on the geometry of the component.
For solvent debinding, commercial products are on the market; certified for use of flammable media like heptane, hexane or acetone. However, water is also used for some binders, e.g., containing PEG. The typical working temperature of the solvent ranges from 40℃ to 60℃. There are also trials using supercritical CO2 for debinding. The motivation is to decrease debinding time and allow larger wall thickness than 10mm of the green part. However, the results remain ambiguous as to whether this process is really beneficial. Finally, it depends on the binder, which has to be adapted to the specific debinding method.
Thermal debinding can take place either in the sintering furnace or in a separate oven. In both cases, the possibility for using sweep gas should be provided to facilitate removal of the binder gas, but also generation of a high vacuum <10-3Pa should be possible. Preferentially the binder residuals are condensed in a chiller behind the furnace. If a separate furnace is used for debinding, the operating temperature should be at least 1000℃ to allow presintering.
The sintering furnace should be a high-vacuum device with metallic heater and shielding. Sintering is the most critical process with regard to contamination due to the high temperature. For titanium, the typical sintering temperature ranges from 1250℃ to 1350℃. However, even higher temperatures up to 1500℃ are necessary, when sintering, for example, intermetallic titanium aluminides.
In addition, in special cases it can be beneficial if the furnace can be run under different gaseous atmospheres, as well. For example, sintering under argon instead of a vacuum may facilitate the avoidance of oxygen pickup, if high-purity gas is used, and sublimation of alloy elements with low melting points can be minimized (e.g., Al). Even sintering under hydrogen partial pressure may be helpful for achieving low oxygen contamination. However, this is associated with higher efforts in respect of equipment and security and the possible formation of hydrides has to be considered.
Recently, furnaces have entered the market focused specifically on titanium processing by optimizing the binder removal. They use special setups like retorts and guiding of the gas flow. If very low contamination is crucial, the purchase of such furnaces dedicated to titanium should be considered.
19.2.4 Porosity
The residual porosity of sintered titanium parts can be more critical than in the case of stainless steel. The reason is the rather high sensitivity of titanium-based materials against notches and small cracks. Especially in the case of fatigue load by stress variation, it is important that the pores are small and as well rounded as possible and surface defects are minimized. In a limited range, these features can be influenced by proper choice of process parameters. Typically, the residual porosity amounts to a value around 3%-4%. This means porosity is closed and an additional hot isostatic pressing (HIP) process can be applied without capsulation, resulting in practically 100% dense material. However, possible impurity uptake has to be considered.
19.2.5 Biocompatibility
Because titanium is a popular material for medical implants, application in this field is also of interest using MIM for processing. In this case, it must be ensured that possible binder residuals do not affect the biocompatibility. In the end, appropriate tests of the sintered components have to prove this. However, it is a good start to use exclusively nontoxic biocompatible binder components. In Section 19.6.1, the current state of this issue is described.
19.3 Basics of processing
19.3.1 Powder handling
The duration of open powder handling, as may occur if feedstock is made in-house, should generally be limited to a minimum. Studies show that for serious oxygen pickup temperatures above 400℃ are necessary (Baril et al., 2010), which is confirmed by the current author's investigations. However, each exposition to air is a risk. In addition, titanium powder is basically burnable and explosive if it is heated to temperatures when fast oxidation occurs (above 400-500℃). As a protection gas, argon is suitable, whereas nitrogen, which is often used as an inert gas in other contexts, is picked up by titanium.
19.3.2 Feedstock production
During kneading binder and powder are heated to temperatures that depend on the specific binder, but usually range from 120℃ to 180℃. As stated previously, temperatures below 200℃ appear to be rather uncritical in terms of oxygen uptake, but in order to guarantee no additional oxygen pickup kneading is done preferably under a protective atmosphere. For kneading, shear roll mixers, double planetary mixers, twin screw mixers or sigma blade kneaders are in use. Their choice depends on the binder and solids loading used. Generally, mixing under high shear is beneficial in order to provide a good homogeneity.
19.3.3 Injection molding
As stated previously, no special equipment is necessary. Process parameters depend on geometry and feedstock. No general advice differing from the processing of stainless steel powders can be given here. The main difference to steel feedstock is the larger powder particle size, as mentioned previously, meaning that powder and binder separate more easily. On the other hand, the smaller difference in density between binder and titanium powder when compared to steel alleviates this effect. The most critical spot is the gate of the mold. The size should be generally greater than in the case of steel powder and great care should be taken concerning edges. A rounded and symmetrically constructed gate positioned in both mold parts appears to be favorable. This is also true for the runner. Today, software simulating the injection molding process can assist significantly to design the mold.
It is very important to make sure that no titanium feedstock is contaminated by other materials, especially steel powder, because brittle or low-melting phases can occur in the microstructure, degrading the mechanical properties significantly. Thus, cleanliness of the machine is critically important. If the feedstock material is changed, it is necessary to clean carefully all parts of the injection molding machine in contact with the feedstock. Furthermore, attention should be paid with respect to the clearance of the back flow valve of the screw. It should be somewhat larger than the maximum size of the powder particle to avoid fretting. In the case of titanium powder, where typically particles smaller than 45 are used, a clearance of 0.1mm between ring of the back flow valve and cylinder wall is usually a reasonable value.
19.3.4 Debinding
During thermal debinding the risk of contamination by carbon is rather high. Therefore, at that time the amount of binder should be as low as possible. This can be influenced by the ratio between first and second binder components. It should be ensured that the entire first component is removed by the first debinding stage (e.g., solvent or catalytic). This results in an open microporous structure, which facilitates the escape of the second component during thermal debinding without cracking of the part. The temperature of the thermal debinding should be as low as possible to minimize the pickup of oxygen and carbon by titanium, as described in Section 19.2.2. Depending on the binder components used, a temperature between 400℃ and 500℃ is usually adequate. Furthermore, as stated previously, some flow of argon gas can be used to support the transport of the binder gases out of the furnace (sweep gas). Using helium is also possible, but more expensive. Nitrogen should not be used, because it reacts with titanium. The debinding ends with heating to sintering temperature. In the case of a separate debinding furnace, the parts are presintered at temperatures around 900℃ for about 1 h. After this treatment, the parts are mechanically rigid, thus they can be handled further for final sintering.
19.3.5 Sintering
Sintering is the most critical production step with regard to contamination by oxygen.In addition, the resulting microstructure is defined by this process and so are the mechanical properties. Therefore, great care has to be taken during sintering of titanium materials.
It is not possible to avoid oxygen pickup completely, but it is possible to minimize it by providing a high vacuum during sintering of at least 10-2 Pa or better. It is also possible to sinter under an atmosphere of high-purity argon, but in this case the sintered density will be lower because of trapping gas in the pores. Furthermore, powder with low oxygen content should be used, for example, the extra low interstitials (ELI) variant of the Ti-6Al-4V alloy. The author's experience is that, if the whole process works well, the difference in oxygen between powder and sintered part can be limited to about 0.05 wt%. However, in practice values up to 0.1 wt% are possible, depending on initial oxygen content of the powder, number of samples in the furnace, and so on.
Choosing the right sintering parameters is always a compromise between low residual porosity and small grains. Generally, high sintering temperature and long hold time lead to higher density, resulting in better strength and ductility. On the other hand, grain coarsening will also be more important, affecting these properties detrimentally. Pure titanium and typical alloys like Ti-6Al-4V are sintered above the beta-transus temperature in the single-phase beta region, which promotes grain growth. As showed it can be beneficial to sinter at lower temperature for a longer time than to hold a higher temperature briefly. However, typical sintering temperature is around 1300℃ combined with a holding time of 2 h. There are statistical tools helping to reduce the number of experiments for optimizing the sintering parameters. Show an example for using the Taguchi method in order to find the best combination of sintering temperature, time, heating rate, and atmosphere for MIM of pure titanium and Ti-6Al-4V, respectively, for a given binder system. Another study of molding, binder and sintering parameters.
The choice of the sintering support is critical, because titanium reacts with nearly all materials. Most MIM titanium providers use Y2O3 or ZrO2 or similar ceramics as supports or as the separating layer.
19.3.6 Further processing
The sintered parts can be surface treated by common polishing methods. Some care should be taken when applying etching techniques because of the surface porosity. Coloring methods like anodic oxidation can also be used as they are usually performed.
HIP can be applied using an argon atmosphere. Typical parameters are 850-915℃ for 2 h at a pressure of 100-200MPa. The density of the sintered parts has to exceed 95% to ensure a closed porosity. Otherwise, HIP is not possible.
19.4 Mechanical properties
Because the mechanical properties are defined by the microstructure, it is important to understand the difference from wrought material. In Fig. 19.2, typical micrographs of wrought and of MIM-processed Ti-6Al-4V alloy are shown. Three obvious differences are visible.
The beta- and alpha-phase morphologies are different. The wrought material shows a globular structure consisting of equiaxed alpha grains with beta-phase at the grain boundary regions. By contrast, the MIM-processed material exhibits a lamellar structure with alpha-beta colonies. This structure is formed during cooling when passing the beta transus temperature.
Grain and colony size, respectively, are significantly different. As mentioned previously, this is due to sintering within the single-phase beta-region. In the case of the wrought material, thermomechanical treatment including recrystallization provides the small grains after the initial billet casting.
The MIM-processed alloy features small isolated pores with preferentially spherical shape. Depending on the sintering parameters the porosity can be influenced, but values below 2%- 3% have barely been realized yet.
All these differences lead to deviations of the mechanical properties from those of the wrought material. The lamellar structure is beneficial with regard to crack growth processes, but strength, ductility and high cycle fatigue properties are generally improved by provision of a fine and globular microstructure. In the following section, typical tensile and fatigue properties of MIM-processed pure titanium and Ti-6Al-V4 are compared with standard values.
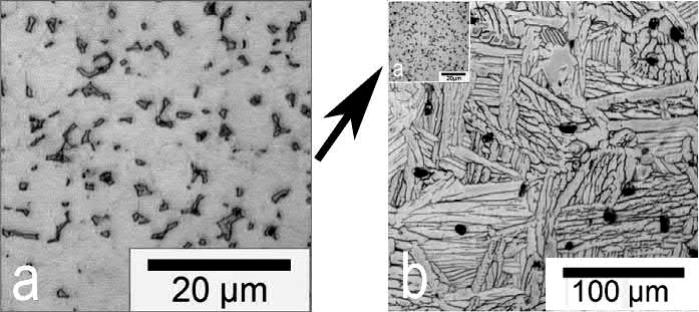
Fig. 19.2 Comparison of typical microstructures of (A) Wrought Ti-6Al-4V. (B) The same material processed by MIM
19.4.1 Tensile properties
Since 2011, a first ASTM standard for MIM-processed Ti-6Al-4V for medical applications has been in existence (ASTM F2885-11). In the year 2013, the standard for MIM of unalloyed titanium (ASTM F2989-13) followed. However, usually the properties of the sintered parts are compared to the limits given by ASTM B348-02, although this standard is intended for wrought material. There are two grades applied for Ti-6Al-4V alloy: Grade 5 and Grade 23, differing primarily in the limit for oxygen. Whereas Grade five allows 0.2 wt% oxygen, Grade 23 (also denoted as ELI) limits this value to 0.13 wt%. In Fig. 19.3, the tensile properties of MIM-processed Ti-6Al-4V are compared to this standard. The values are taken from three different published studies, revealing the status today, when the oxygen level is kept well below 0.3 wt%. In addition, a comparison is shown between as-sintered samples and samples exposed to an additional HIP process after sintering is done. The lighter regions of the bars represent the scatter of the values measured in the studies. Meanwhile, there is a number of more recent studies revealing, that excellent tensile properties are rather easily achievable today.
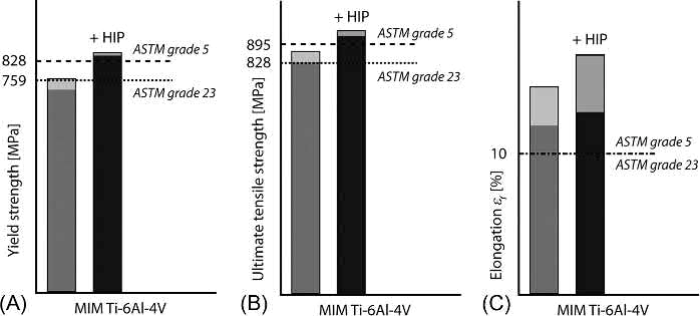
Fig. 19.3 Typical tensile properties (A: yield strength; B: ultimate tensile strength; C: elongation to fracture) of MIM-processed Ti-6Al-4V alloy in comparison to ASTM standards B348-02, Grade 5 and Grade 23. Additionally, the properties after an additional HIP process are shown
For powders, either Grade 23 prealloyed powder or blending of elementary and prealloyed powder was used. Furthermore, different binders and processing temperatures were applied in the studies. However, the results are very similar. By proper processing, the tensile properties can fulfill the strength requirements for Grade 23. Closing of the porosity increases the strength so strongly that even Grade 5 requirements are met. In all cases, the ductility of the specimens is significantly higher than the standards demand. This is a sign that the amount of interstitials is kept under certain limits. In two of the cited studies, oxygen contents between 0.20 wt% and 0.23 wt% were gained. It is also obvious that between the studies the scatter of elongation is significantly larger than that of strength. This again proves the difficulty and the need to control the processes very accurately with regard to oxygen and carbon pickup. On the other hand, investigations showed that the tensile properties do not deteriorate, if the oxygen equivalent according to Eq. (19.1) remains below around 0.4 wt%. As a matter of fact, in that study an oxygen equivalent of 0.37 wt% led to yield strength of 784MPa, ultimate tensile strength of 901MPa and elongation to fracture of 14.8%. In this case, the oxygen content was 0.26 wt %. Figs. 19.4 and 19.5 show the results for tensile strength and plastic elongation in relation to the interstitial equivalent.
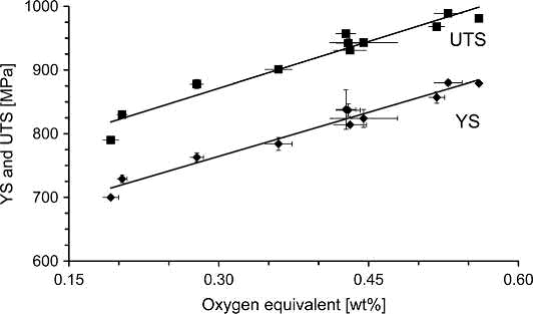
Fig. 19.4 Dependence of yield strength (YS) and ultimate tensile strength (UTS) on oxygen equivalent, measured on MIM-processed Ti-6Al-4V samples
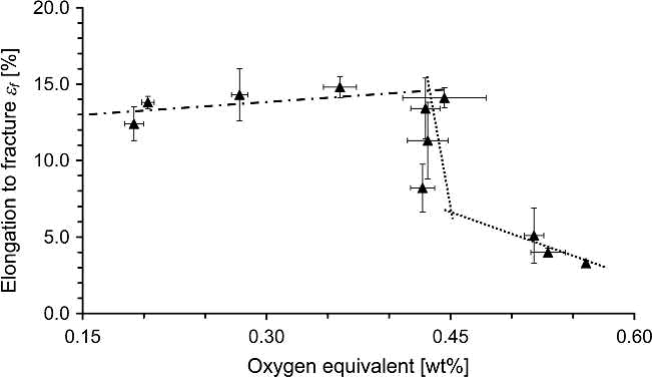
Fig. 19.5 Dependence of plastic elongation of MIM-processed Ti-6Al-4V on oxygen equivalent
Evaluated the data from all studies on MIM of Ti-6Al-4V today with regard to oxygen equivalent and tensile properties. Concluded that Oeq should not exceed 0.34 wt% and the porosity should be kept under 3% to match the mechanical requirements for surgical implants. This result and also a further study are in accordance with the present author's study if a certain security margin is considered.
Today, there is quite consent among scientists that oxygen can be used as an intended alloying element to increase the mechanical properties. This is at least true for the tensile properties. First studies on the influence of oxygen on high cycle fatigue indicate a loss of fatigue strength with increasing oxygen content (Amherd Hidalgo et al., 2016). However, different to the tensile properties no sudden and dramatic decrease appears in the case of fatigue.
Such an apparent independence of elongation within a certain range of interstitials as visible in Fig. 19.5 was not detected in the case of pure titanium. In their study, an increase of oxygen content from 0.2 wt% to 0.4 wt% meant a linear loss of ductility from 20% to 8% while UTS increased from 560 to 720MPa. On the other hand, this study shows that even pure titanium is processable by MIM and good mechanical properties can be achieved if proper raw material is used. Meanwhile, further improvement could be achieved. In this study, elongation up to 27% was achieved in combination with around 470MPa tensile strength.
Results from MIM processing of other alloys like, for example, Ti-Al-7Nb exist too. One overview of mechanical properties is given. However, it is rather difficult to compare these studies, because different powders, binders and processing parameters were used. Some samples were even additionally applied to a HIP process. Figs. 19.6 and 19.7 display the large scatter of ultimate tensile strength and elongation resulting from these studies performed on unalloyed titanium, Ti-6Al-4V and Ti-6Al-7Nb. The diagrams make clear how important are proper processing and adequate powders for guaranteeing high quality and reproducible production. Well-balanced combinations of ultimate tensile strength and elongation in these studies were 640MPa /20% for unalloyed titanium and 830MPa/11% for Ti-6Al-7Nb. As shown before, in the case of Ti-6Al-4V, even >14% elongation combined with 900MPa ultimate tensile strength is possible. These results agree to a newer study on MIM of Ti-6Al-7Nb.
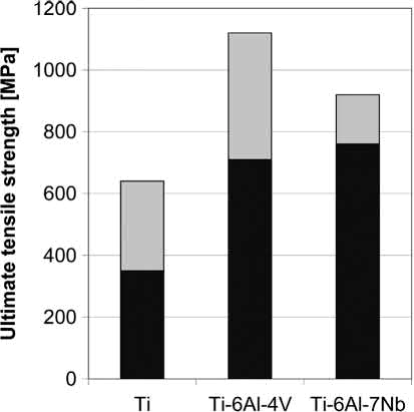
Fig. 19.6 Range of ultimate tensile strength according to experiments collected for unalloyed titanium, Ti-6Al-4V and Ti-6Al-7Nb. All samples were produced by MIM, but using different powders, binders and process parameters. Some samples were additionally subjected to a HIP process.
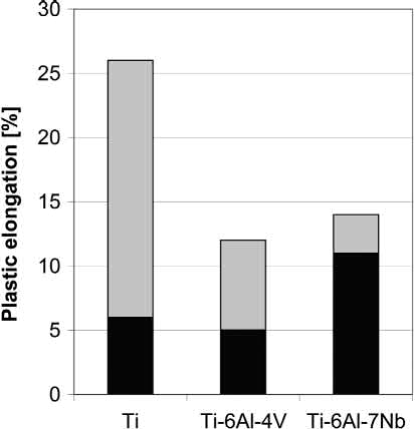
Fig. 19.7 Range of elongation according to the experiments collected
The studies show that it is possible to receive highly ductile components with reasonable strength comparable to wrought material. However, if optimal tensile properties are required, closing of the pores by a HIP process is mandatory.
19.4.2 Fatigue properties
Studies on fatigue of MIM-processed titanium materials are rather rare. Ti-6Al-4V was investigated with regard to fatigue under bending and axial load, respectively. In both cases, the endurance limit defined for 107 cycles was between 350MPa and 400MPa for the as-sintered samples. Muterlle found similar fatigue resistance in a study about the effect of shot peening. Thus, the fatigue properties of MIM-processed titanium materials are better than those of cast, but inferior to those of wrought material. In the latter case, there is a large scatter of values which can be found in the literature depending strongly on the actual microstructure. A range between 450MPa and 800MPa can be assumed for wrought Ti-6Al-4V. The reason for the lower endurance limit of MIM Ti-6Al-4V alloy was studied in depth in. It is shown that, first, as typical for fatigue behaviour, the surface is essential. For example, by simple shot peening the endurance limit can be improved by around 50MPa due to surface smoothing and introduction of compressive stress in the surface near region. Starting sites for crack growth are the pores and defects on the surface. Therefore, the fatigue behaviour can also be influenced by injection molding parameters and binder.
Interestingly, closing of the residual porosity has a much greater effect on the tensile properties than on the fatigue properties. The best configuration investigated and exposed to an additional HIP process led to an endurance limit of 500MPa, meaning the lower end of wrought material. On the other hand, the size of the microstructure turned out to be the most crucial parameter after surface properties. As pointed out previously, the grain size of the MIM microstructure is typically much larger than that of wrought material. Other studies show the strong dependence of the fatigue strength on the grain size, too. A Hall-Petch relationship between these two parameters was detected. Fine powders were used in this study to lower the sintering temperature in order to achieve smaller grains.
In order to generate a finer microstructure of MIM Ti-6Al-4V, 0.5 wt% of elementary boron powder was added to the alloy powder. During sintering needle-shaped TiB was formed, which is known as a grain refiner during casting. In fact, the addition of boron effects a drastic change of the microstructure as shown in Fig. 19.8. In order to identify the actual grains more clearly in addition to SEM images backscattered electrons (BSE) mode, electron backscatter diffraction (EBSD) measurements were made. Each gray tone represents one specific grain orientation. It is obvious that the microstructure of the boron-added alloy is completely different. Only few lamellar regions are visible and the grain size changed from around 150-20μm. In addition, the boron addition improves the sintering process resulting in a residual porosity of only 2.3%. The finer microstructure effects an increase in fatigue strength to 640MPa, which is equivalent to that of high performance wrought material. Yield stress and UTS amount to 787MPa and 902MPa, respectively, in connection with a plastic elongation of 11.8%. This means all mechanical requirements for Grade 23 are fulfilled by this material. This example shows that it is worthwhile to develop specific alloys optimized for MIM processing as has long been practiced in the case of materials intended to be processed by casting or forging.
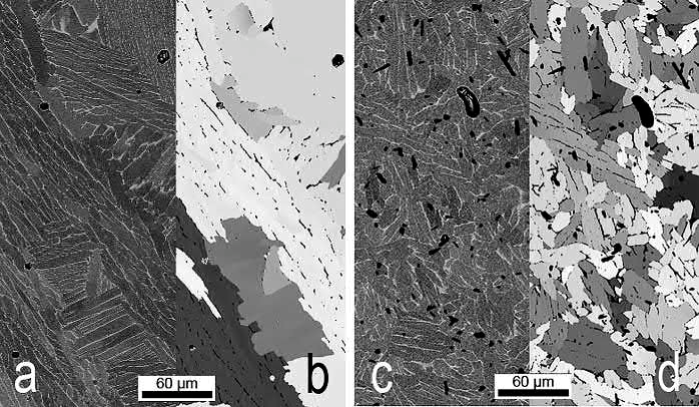
Fig. 19.8 Microstructures of MIM-processed specimens: (A) and (B) Ti-6Al-4V; (C) and (D) Ti-6l-4V-0.5B. (A) and (C) Images are made by scanning electron microscopy (SEM) in BSE mode. (B) and (D) images are evaluated by electron backscatter diffraction (EBSD), the same gray scale means the same grain orientation. The alpha phase is shown.











